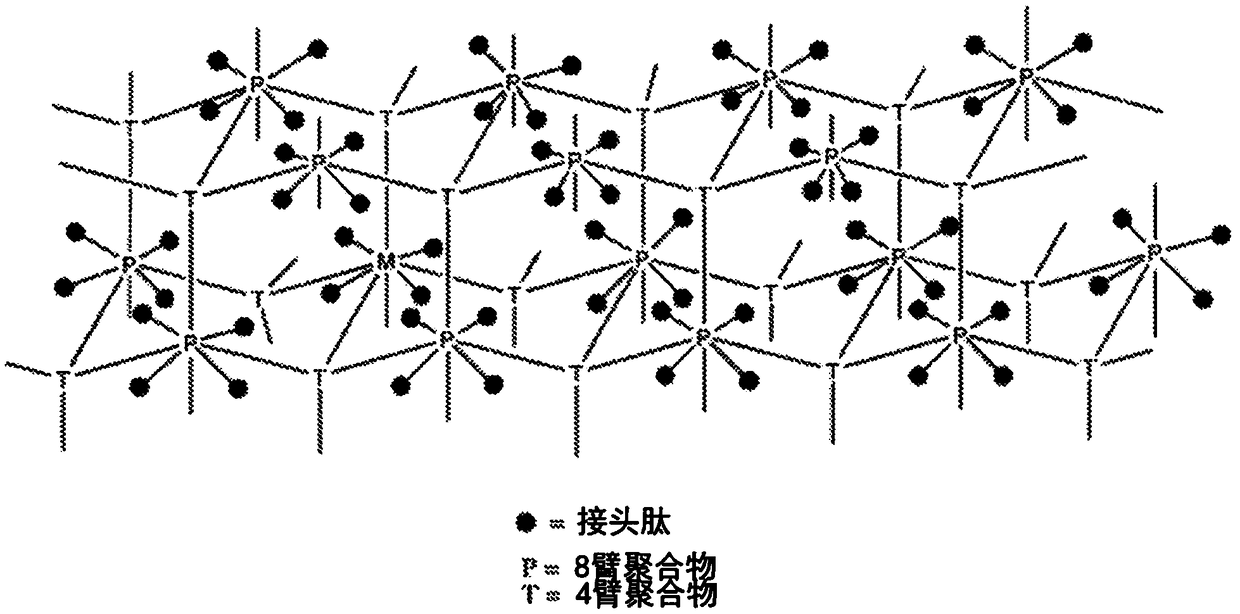
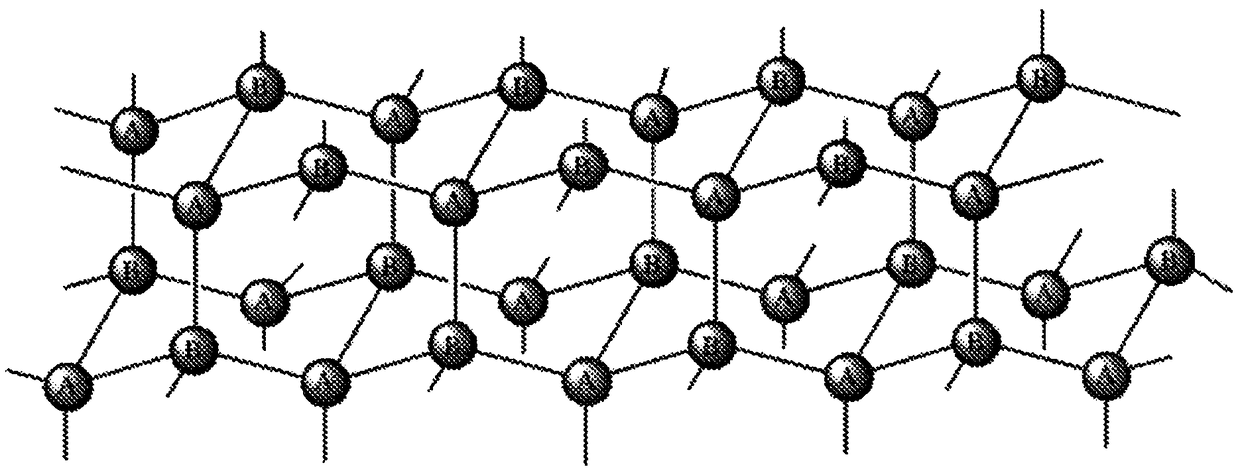
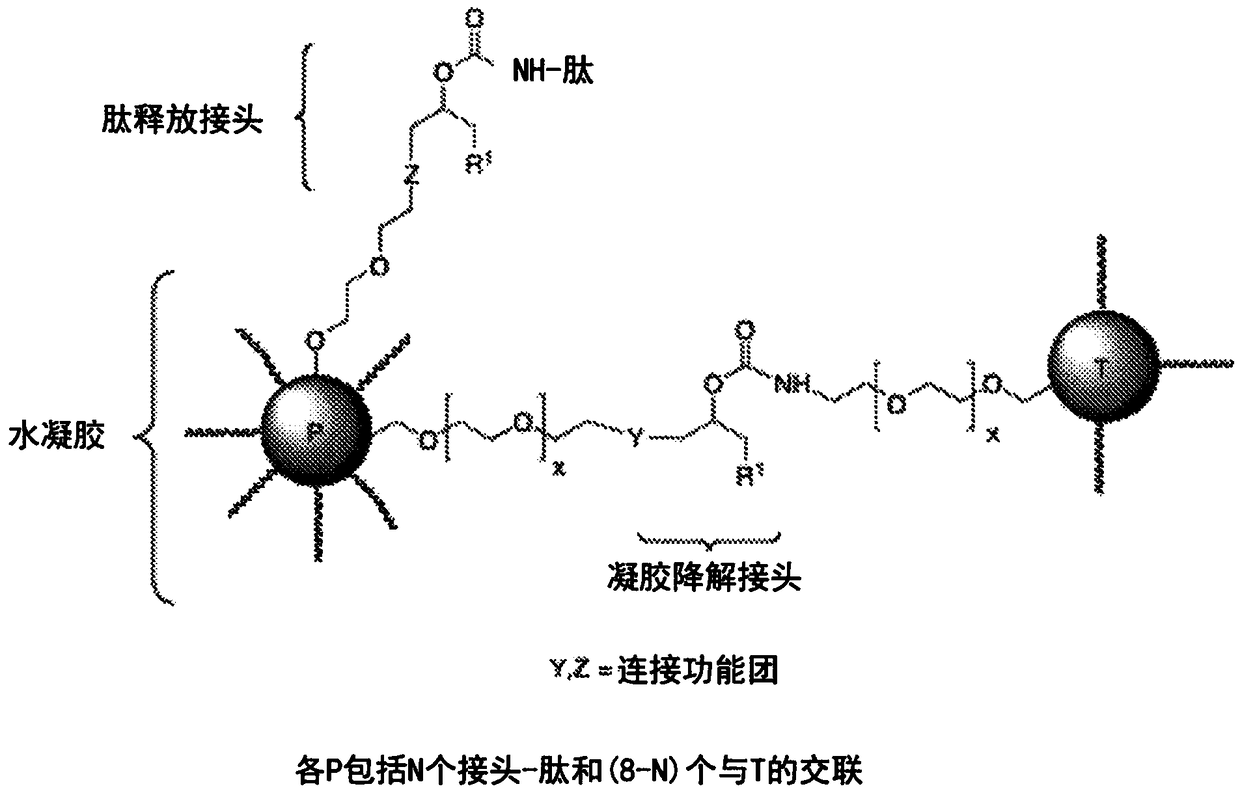
Extended release conjugates of exenatide analogs
A conjugate, sustained-release technology, applied in the direction of hormone peptides, specific peptides, drug combinations, etc., can solve problems such as instability of the original mixture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0239] Preparation of Azido-Linker-[N28Q]Exenatide of Formula (4)
[0240] Among them, R 1 = CN or MeSO 2 ; 2 = H; an R 5 = H and other R 5 =(CH 2 ) 5 N 3 ;P=N α -[N28Q] Exenatide
[0241] use Rink amide resin (0.5 meq / g) in Peptides were synthesized by standard solid-phase methods on a peptide synthesizer. Fmoc-amino acid (5 equiv / coupling) to the N-terminus of the peptide chain using HCTU (4.9 equiv / coupling) and N,N-diisopropylethylamine (10 equiv / coupling) in DMF at ambient temperature Double-couple. The Fmoc group was removed using 20% 4-methylpiperidine in DMF. [N28Q]Exenatide was deprotected and cleaved from the resin using 95:2.5:2.5 trifluoroacetic acid / triisopropylsilane / dithiothreitol.
[0242] Use the PeakScientific Shimadzu for 5μC18 column (50x 20mm ID) TM Crude [N28Q]exenatide (22 mg) was purified on a semi-preparative scale by the LC-20AD system using a linear gradient of 30%-60% MeCN (0.1% TFA) in water (0.1% TFA) elution. The purest ...
Embodiment 2
[0248] Preparation of PEG Hydrogel Microspheres
[0249] Using 2 reagents (2-reagent) Hydrophobic flow focusing microfluidic chip (Dolomite) with 7 parallel 50um droplet forming channels. Fluids are controlled by a pneumatically driven pump that functions similarly to the Miltos pressure pump made by Dolomite Microfluidics Dolomite. These pumps use compressed air to drive liquids through microfluidic chips. The drive pressure was computer controlled using an appropriate pressure regulator (MPV series from ProportionAir) to maintain a steady flow rate by using a feedback loop from a liquid flow sensor (SLI-0430 from Sensirion). This type of fluid control is scalable to deliver fluids from multi-liter reservoirs, and handles flow rates with about 1% standard error, which is better than syringe pumps that typically have as much as 20% oscillation in their flow rates. The system was used to deliver both hydrogel prepolymer solutions as well as the continuous phase. Typical fl...
Embodiment 3
[0254] Preparation of cyclooctyne-microspheres
[0255] The reaction was carried out in a syringe reaction vessel as shown below. To each 4 mL amino-microsphere packing suspension in MeCN containing 2 μmol amine / mL was added 32 μmol DIPEA (4 equiv) in 1 mL MeCN, and 9.6 μmol (1.2 equiv) of 1-fluoro-2- Pentafluorophenyl cyclooctyne-1-carboxylate (MFCO-PFP). After 1 hour of shaking at ambient temperature, a small amount (~50 uL) of microspheres was expelled from the outlet of the syringe and treated with 0.5 mL of 0.04% w / v TNBS in 0.1 M sodium borate (pH ~9.3) (1) for 30 min; The initial amino-microspheres (with a dark orange color) compared to the microsphere color matched the TNBS solution to indicate a complete reaction. After the reaction, by adding 8 μmol (1 eq.) of Ac in 1 mL of MeCN 2 O for 10 minutes to cap the microspheres. After removing the supernatant, approximately 2 mL of microspheres were transferred to a second 10 mL syringe, each slurry was washed with 4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com