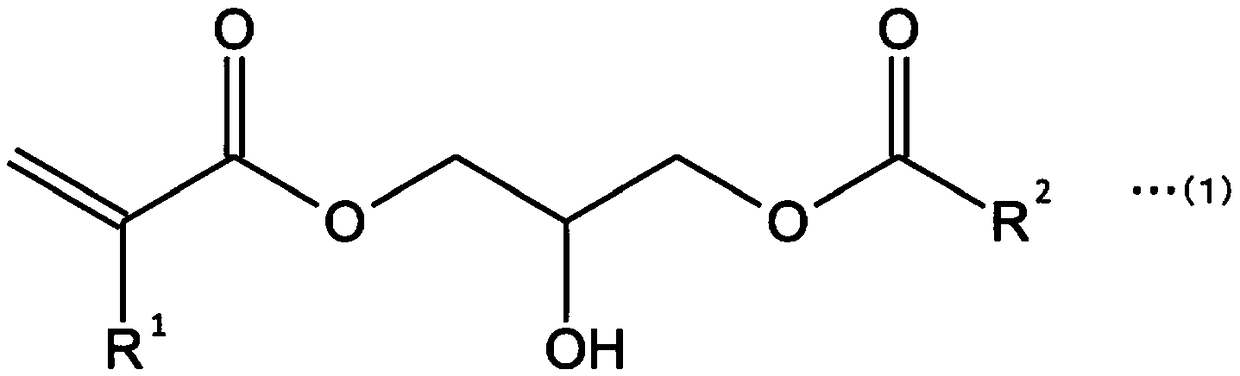
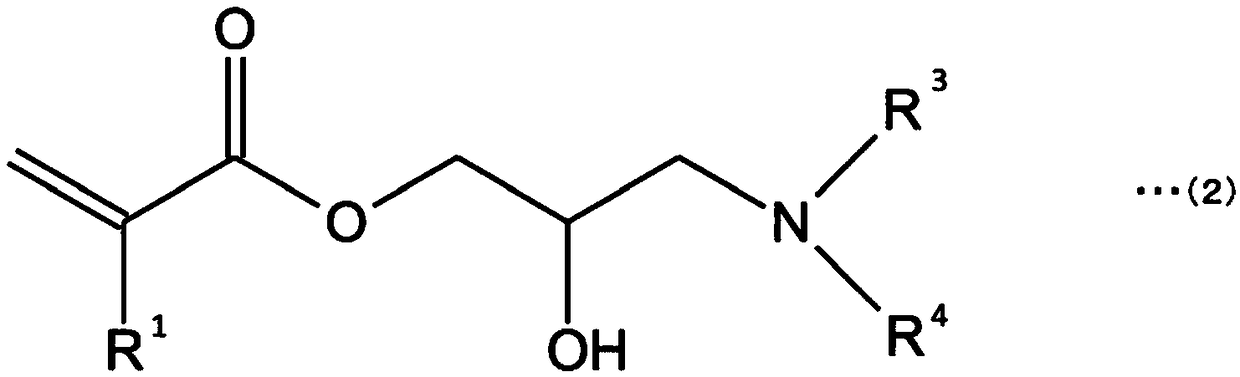
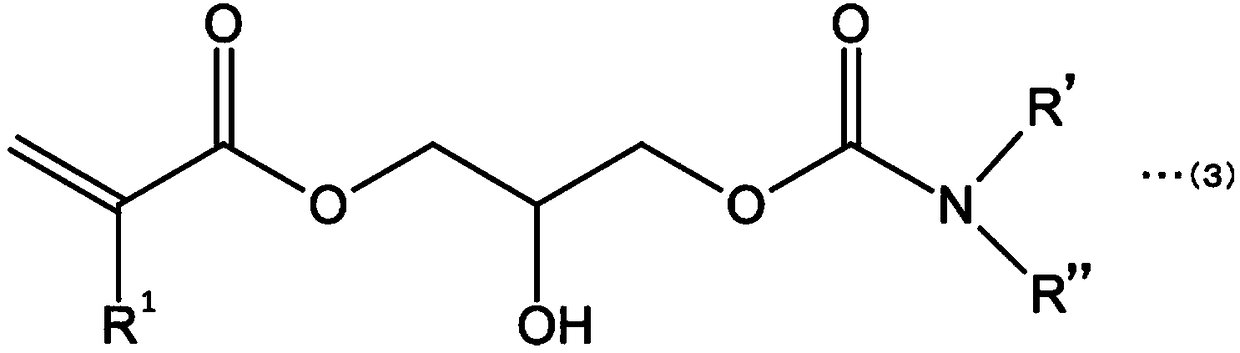
Curable composition and product
A technology for curable compositions and cured products, applied in the field of curable compositions and products, can solve the problems of time-consuming, brittle, and hardened products, and achieve the effect of ensuring design freedom
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0180] Hereinafter, an Example is given and it demonstrates more concretely. It should be noted that these Examples are illustrative and should not be construed as limiting, which is self-evident.
Synthetic example 1
[0181] (Synthesis example 1) GMA decanoic acid
[0182] Add 4.00 g (28.1 mmol) of glycidyl methacrylate (product name "LIGHTESTER G", manufactured by Kyoeisha Science Co., Ltd.), 4.85 g (28.1 mmol) of capric acid, and 0.074 g (0.28 mmol) of triphenylphosphine as a catalyst. , and allowed to react for 24 hours at 60°C. A light brown compound (GMADAc) mainly composed of 2-hydroxy-3-decanoyloxypropyl methacrylate was obtained. From the results of the IR spectrum measurement of the compound, it was confirmed that the -OH stretching (broad peak, 3300 to 2500 cm- 1 ) absorption disappears. In addition, it was confirmed that -OH stretching (broad peak, 3300 to 3600 cm- 1 )peak.
[0183] It should be noted that, in the above synthesis reaction, the epoxy group of glycidyl methacrylate is ring-opened, and is bonded with an unsaturated carboxylic acid to form an ester bond. The ring-opening occurs at both the α-position and the β-position, and the α-adduct formed at the α-position ...
Synthetic example 2
[0184] (Synthesis Example 2) GMA 2-ethylhexanoic acid
[0185] Add 5.00 g (35.2 mmol) of glycidyl methacrylate, 5.07 g (35.2 mmol) of 2-ethylhexanoic acid, and 0.092 g (0.35 mmol) of triphenylphosphine as a catalyst, and react at 60°C for 24 hours . A dark brown compound (GMA2EHAc) mainly composed of 2-hydroxy-3-(2-ethylhexanoyloxy)propyl methacrylate was obtained. From the results of the IR spectrum measurement of the compound, it was confirmed that the -OH stretching (broad peak, 3300 to 2500 cm- 1 ) absorption disappears. In addition, it was confirmed that -OH stretching (broad peak, 3300 to 3600 cm- 1 )peak.
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com