Phosphorylation modification-based photocatalytic material and preparation and application methods thereof
A phosphorylation and photocatalyst technology, applied in chemical instruments and methods, ammonia preparation/separation, physical/chemical process catalysts, etc., can solve problems such as increasing reaction costs, and achieve the effect of simple preparation and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Phosphorylated LaFeO with Photocatalytic Nitrogen Fixation Function 3 The preparation method is as follows:
[0064] (1) Take 1.5mmol of ferric nitrate, 1.5mmol of lanthanum nitrate and 3mmol of citric acid and dissolve them in 35ml of deionized water, and stir evenly with a magnetic stirrer at room temperature until it dissolves completely;
[0065] (2) configure 2M NaOH solution, and use it to adjust the pH value of the above solution to about 9;
[0066] (3) Place the precipitated powder in a muffle furnace, keep it warm at 650°C for 3 hours, and finally cool down to obtain a powder sample;
[0067] (4) Immerse the powder sample in 0.2M phosphoric acid and stir for 1 hour;
[0068] (5) After the powder is filtered out, it is kept at 300°C for 2 hours, and a second heat treatment is performed to obtain phosphorylated LaFeO 3 .
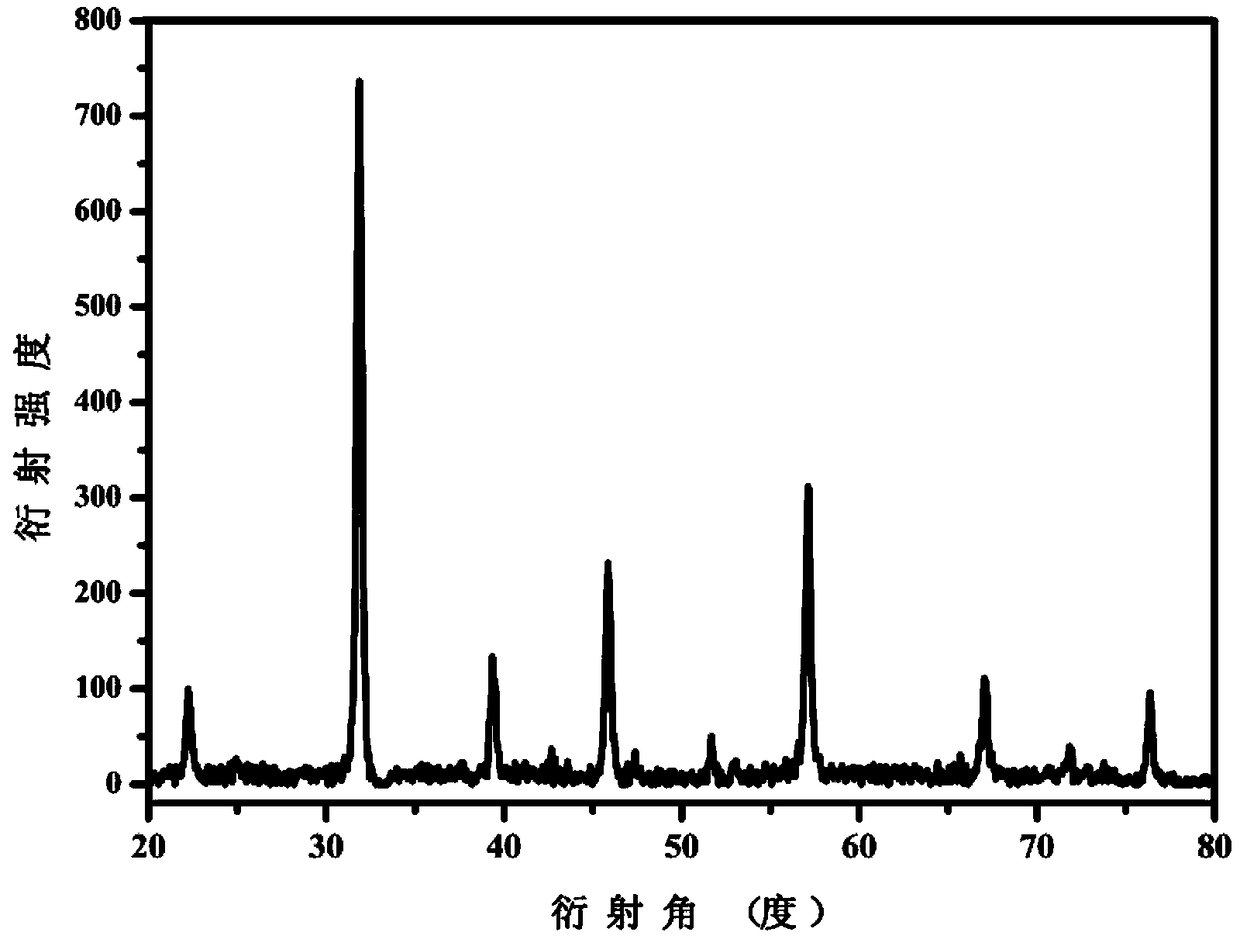
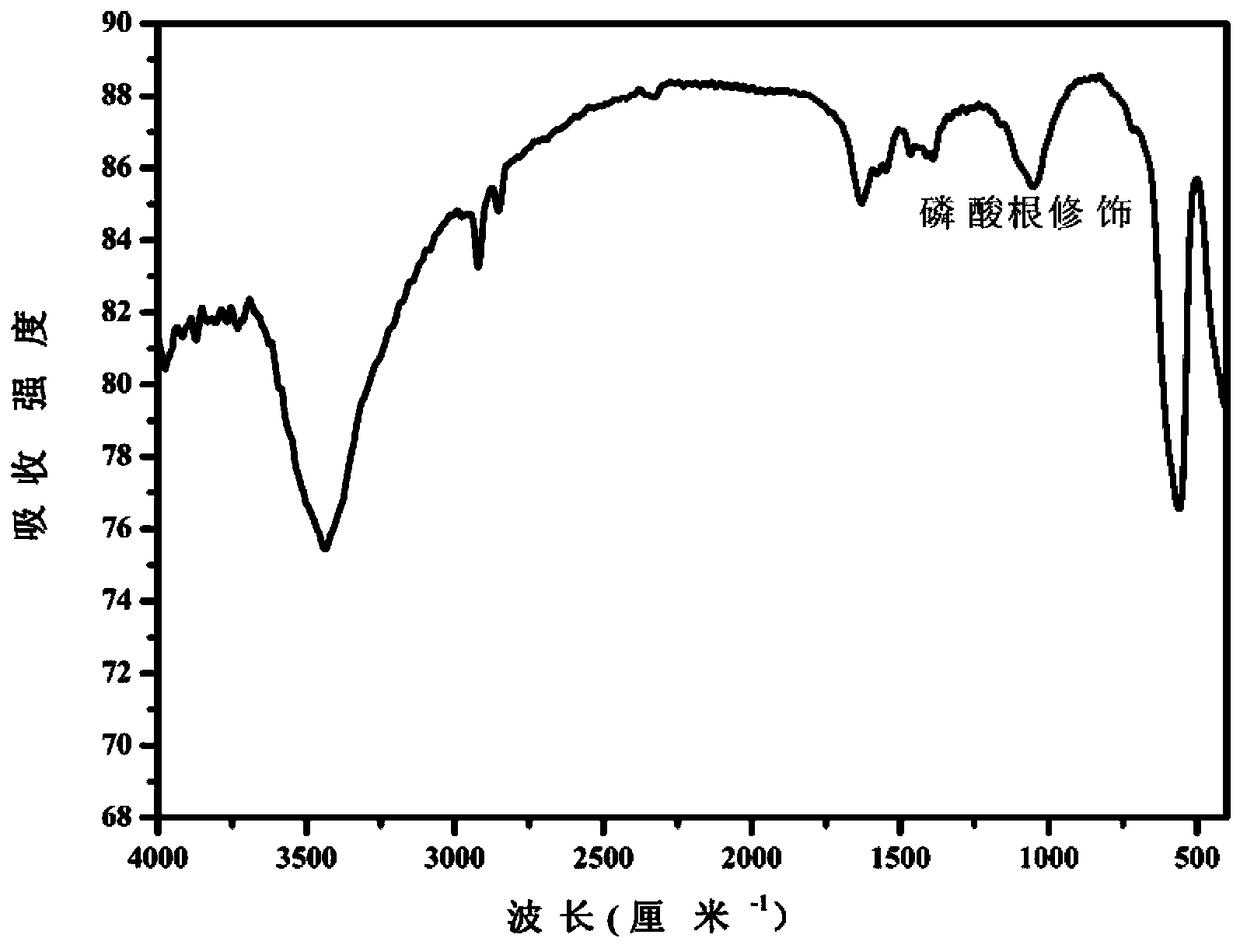
[0069] Phosphorylated LaFeO prepared in embodiment 1 3 To characterize. Characterized by XRD, the resulting phosphorylated LaFeO 3 The...
Embodiment 2
[0071] Phosphorylated LaFeO obtained in Example 1 3 Performing Ammonia Characterization
[0072] The experimental steps include: 1. Disperse 0.1 g of the photocatalyst prepared in Example 1 in the reactor. 2. Feed N into the reactor 2 / H 2 O mixed gas, the gas flow rate is 150mL / min, N 2 / H 2 The molar ratio of O is 100:1, and the total amount of gas introduced is 9L. 3. Use a 500W xenon lamp to irradiate the photocatalyst in the reactor to cause a photocatalytic reaction. 4. Use 0.2mmol acidic solution as NH 3 The absorption solution, according to Nessler's reagent spectrophotometry for NH 3 Concentration detection. Figure 5 Shown is phosphorylated LaFeO 3 Ammonia production-time diagram. from Figure 5 It can be seen that the ammonia yield exhibited a linear increase with a yield of 100 μmol / g h.
Embodiment 3
[0078] Phosphorylated LaFeO with Photocatalytic Nitrogen Fixation Function 3 The preparation method is as follows:
[0079] (1) Weigh 1.5 mmol of iron acetylacetonate, 1.5 mmol of lanthanum acetate and 3 mmol of citric acid and dissolve them in 40 ml of deionized water, and stir evenly with a magnetic stirrer at room temperature until they are completely dissolved;
[0080] (2) Configure 2M KOH solution, and use it to adjust the pH value of the above solution to about 8;
[0081] (3) Place the precipitated powder in a muffle furnace, keep it warm at 650°C for 3 hours, and finally cool down to obtain a powder sample;
[0082] (4) Dip the powder sample in 0.2M phosphoric acid and stir for 3 hours;
[0083] (5) After the powder is filtered out, it is kept at 500°C for 2 hours, and a second heat treatment is performed to obtain phosphorylated LaFeO 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com