A kind of catalyst for preparing isooctanoic acid by oxidation of isooctylaldehyde and preparation method thereof, and method for preparing isooctanoic acid
A technology of isooctaldehyde catalysis and catalyst, which is applied in the direction of physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc. Separation and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
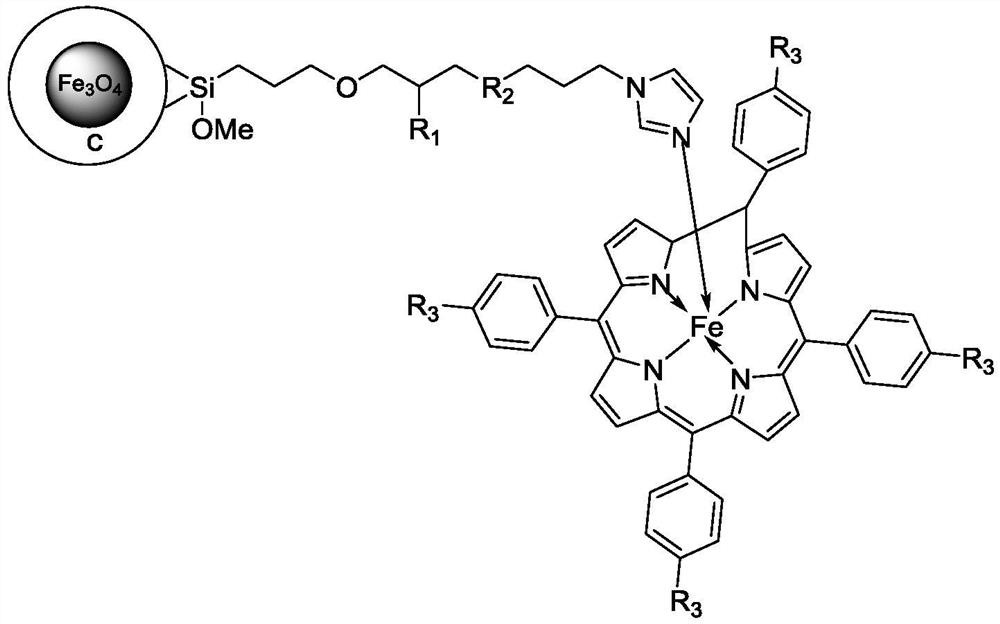
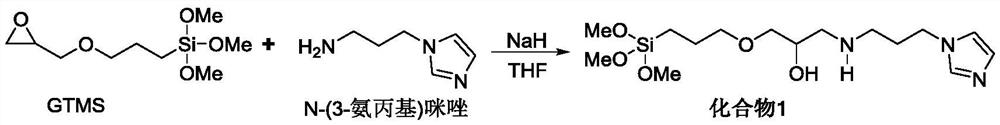
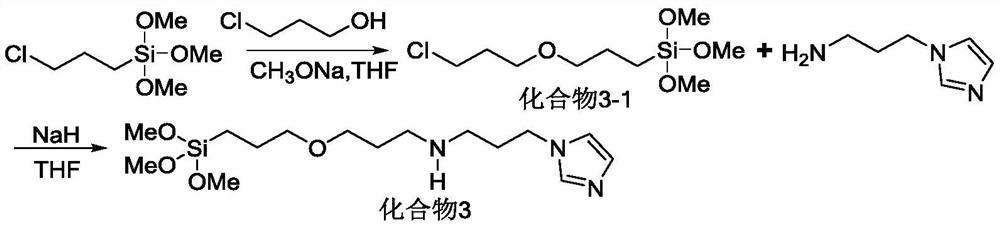
[0054] 5,10,15,20-(tetracarboxymethyl phenyl)porphyrin (2g, 2.4mmol) and ferrous chloride tetrahydrate (2.38g, 12mmol) were dissolved in 60mL DMF solution, and after reflux for 12h, vacuum DMF was distilled off under reduced pressure. After cooling to room temperature, pour 60 mL of deionized water into the round bottom flask, stir for 40 min at room temperature, then filter with suction, wash with water three times, and obtain R after drying. 3 Iron porphyrin IV of =-COOMe.
Embodiment 2
[0056] Referring to the method of Example 1, 5,10,15,20-(tetranitrophenyl)porphyrin (1.59g, 2mmol) was reacted with ferrous chloride tetrahydrate (1.99g, 10mmol) to prepare R 3 =-NO 2 The iron porphyrin compound IV.
Embodiment 3
[0058] 5,10,15,20-(tetracarboxymethylphenyl)porphyrin (2g, 2.4mmol) and hydrazine hydrate N 2 h 4 ·H 2 O150mL was dissolved in 200mL DMF, heated to reflux for 10h, cooled to room temperature, and then 300mL deionized water was added. Stir at room temperature for 1 h, then filter with suction, wash with water three times, and dry to obtain a purple solid powder, which is R 3 =-CONHNH 2 Compound III. Will R 3 =-CONHNH 2 Compound III (1.7g, 2mmol) and ferrous chloride tetrahydrate (2.78g, 14mmol) were dissolved in 50mL of DMF, and refluxed for 12h. After the reaction was completed, the DMF was distilled off under reduced pressure, washed with water three times, and the organic phase was washed with anhydrous sulfuric acid. Magnesium was dried and distilled under reduced pressure to obtain R 3 =-CONHNH 2 The iron porphyrin compound IV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com