Preparation method and device of nitrogen oxide
A technology of nitrogen oxides and oxygen, which is applied in the field of nitrogen oxides preparation, can solve problems such as unfavorable project stable operation, serious material corrosion, unsuitable application of post-processing plants, etc., achieve continuous and stable supply and use, easy reaction, and benefit The effect of continuity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
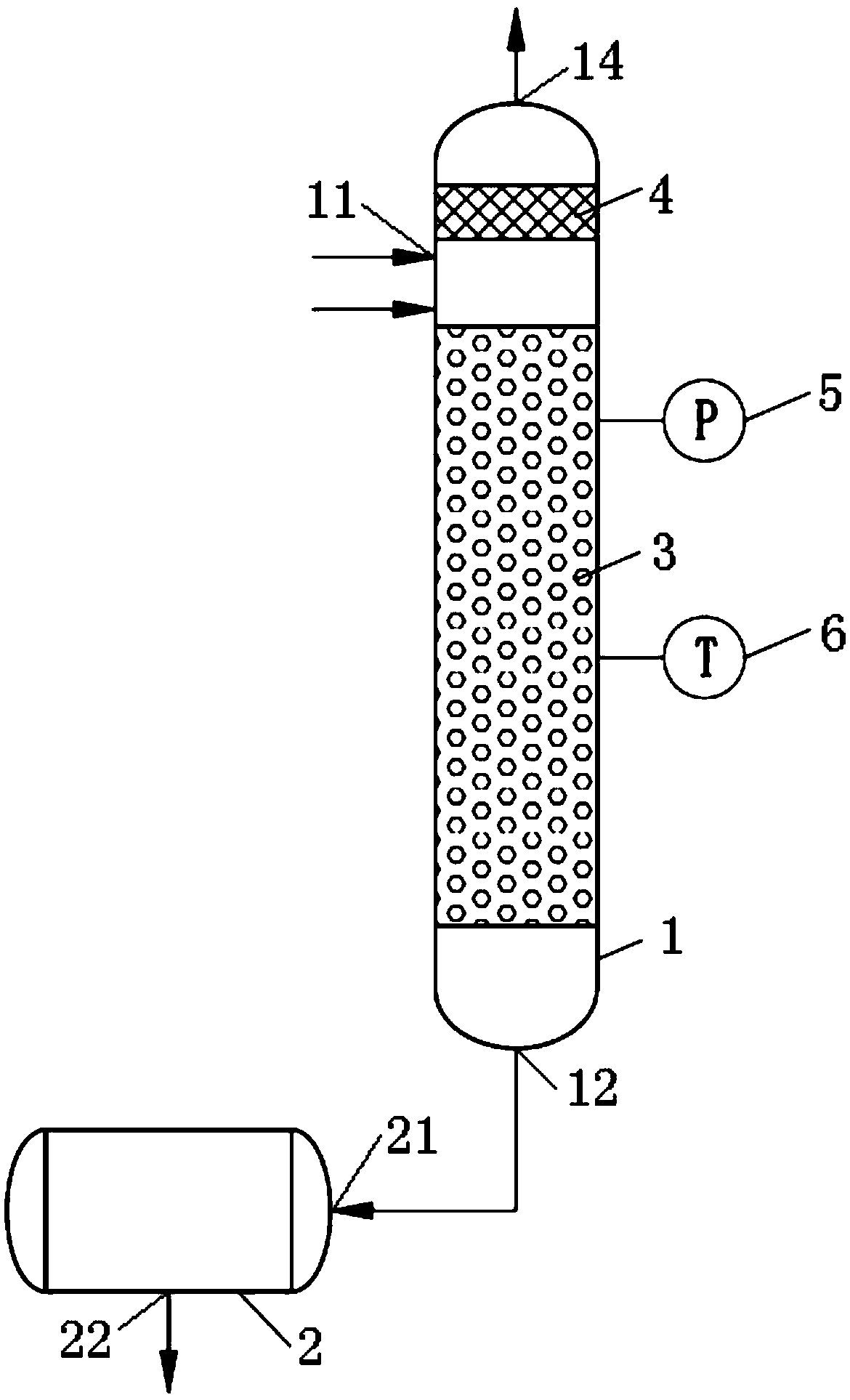
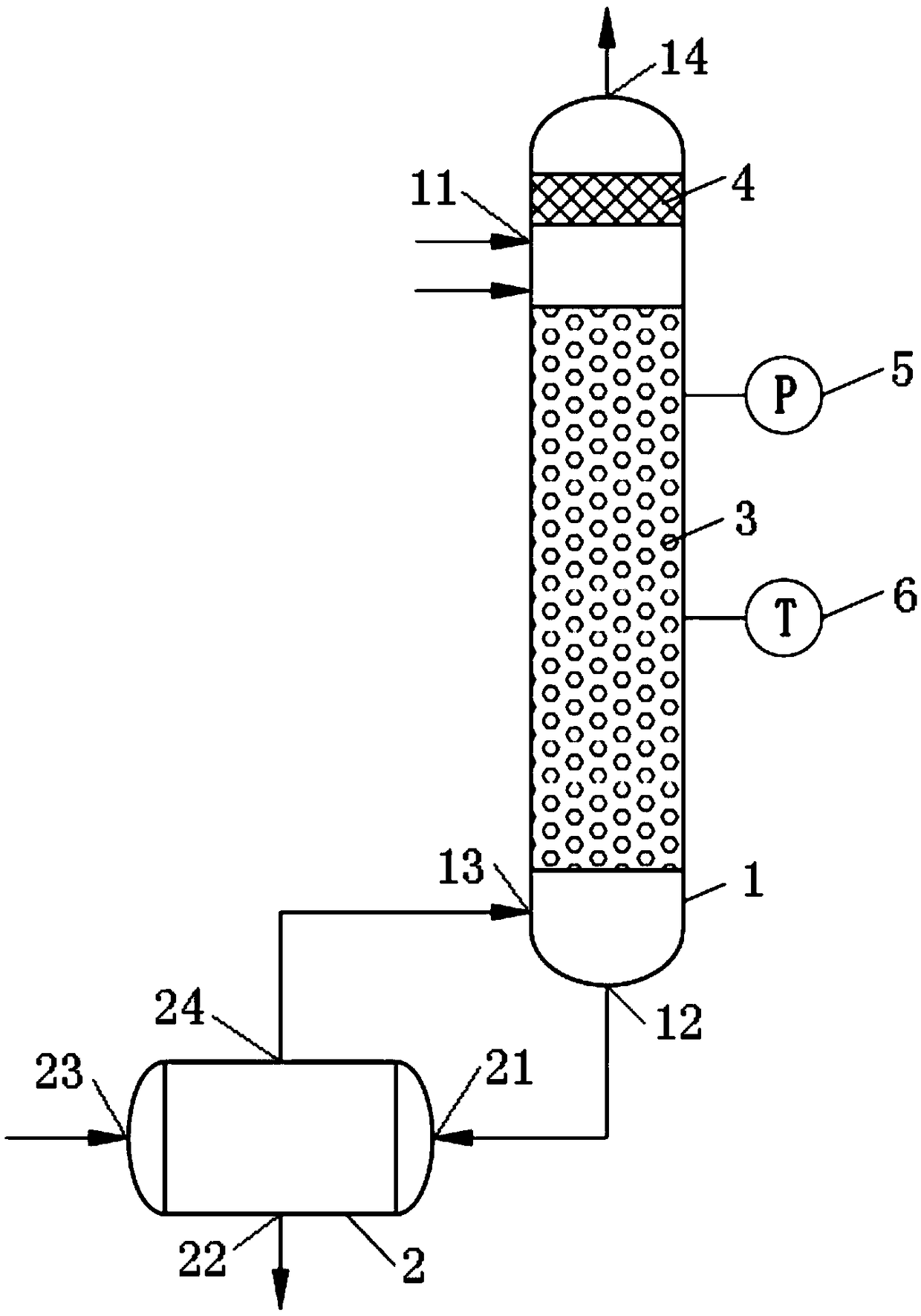
[0030] Such as figure 1 As shown, the device used in the preparation method of nitrogen oxides in this embodiment includes:
[0031] Reaction column 1, reaction column 1 is equipped with filler 3, preheated nitric acid solution and preheated nitrite solution are passed into reaction column 1, mixed in filler 3, nitric acid and nitrite react to generate nitrogen oxide gas , nitrogen oxides are NO x , where 1<x<2. ;
[0032] The buffer tank 2 includes: a first inlet 21 of the buffer tank, a first outlet 22 of the buffer tank, the first inlet 21 of the buffer tank is connected to the outlet 12 of the reaction column, and the liquid flowing through the filler 3 flows into the buffer tank through the first inlet 21 of the buffer tank The tank 2 is buffered, and the liquid in the buffer tank 2 is discharged from the first outlet 22 of the buffer tank.
[0033] The present embodiment provides the preparation method of nitrogen oxides carried out using the above-mentioned device, ...
Embodiment 2
[0037] This embodiment provides a method for preparing nitrogen oxides using the device in Embodiment 1, comprising the following steps:
[0038] 1) Preheat 11.5mol / L nitric acid solution and 3mol / L sodium nitrite solution to 45°C;
[0039] 2) Pass nitric acid solution and sodium nitrite solution into reaction column 1 with a flow rate of 50L / h and 50L / h respectively, the molar ratio of nitric acid and sodium nitrite in reaction column 1 is 11.5:3, and in reaction column 1 The filler 3 is fully contacted to react, the reaction temperature is 45°C, and nitrogen oxide gas is generated, and nitrogen oxide is NO x , where, 1<x<2;
[0040] 3) The nitrogen oxides produced by the reaction pass through the demister 4 to remove entrained liquid droplets to obtain nitrogen oxide products with a pressure of 3 atmospheres, which can be directly used in subsequent oxidation reactions.
[0041] According to the analysis of nitrogen oxide products, the output of nitrogen dioxide in nitroge...
Embodiment 3
[0043] This embodiment provides a method for preparing nitrogen oxides using the device in Embodiment 1, comprising the following steps:
[0044] 1) Preheat 8mol / L nitric acid solution and 1mol / L potassium nitrite solution to 40°C;
[0045] 2) Pass nitric acid solution and potassium nitrite solution into reaction column 1 with a flow rate of 10L / h and 20L / h respectively, the molar ratio of nitric acid and potassium nitrite in reaction column 1 is 4:1, and in reaction column 1 The filler 3 is fully contacted to react, the reaction temperature is 40°C, and nitrogen oxide gas is generated, and nitrogen oxide is NO x , where, 1<x<2;
[0046] 3) The nitrogen oxides generated by the reaction pass through the demister 4 to remove entrained liquid droplets to obtain nitrogen oxide products with a pressure of 2 atmospheres, which can be directly used in subsequent oxidation reactions.
[0047] According to the analysis of nitrogen oxide products, the output of nitrogen dioxide in nit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com