RBP2 enzyme inhibitor small molecular compound WXSA-072A as well as preparation method and anti-gastric cancer application thereof
A technology of WXSA-072A and small molecule compounds, which is applied in small molecule compounds of RBP2 enzyme inhibitors and anti-gastric cancer applications, can solve the problems of poor cell permeability, lack of selectivity, complicated interpretation of biological research, etc., and achieve migration ability Reduced, novel structure, and the effect of inhibiting RBP2 enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
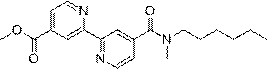
[0034] The preparation of embodiment 1 WXSA-072A
[0035] One, the preparation of intermediate 2
[0036] Weigh (2,2'-bipyridyl)-4,4'-dicarboxylic acid dimethyl ester (0.55 g, 2.0 mmol) into a conical flask, add tetrahydrofuran:methanol=1:1 total 40mL, stir at room temperature for 15 Minutes; then 2 mL of 1 mol / L potassium hydroxide aqueous solution was added dropwise into the Erlenmeyer flask under an ice bath, and continued stirring at room temperature overnight. After the reaction was completed, the solvent was evaporated to dryness under reduced pressure, 50 mL of water was added thereto, and then 1 mol / L of dilute hydrochloric acid was added until the solid was precipitated, the filter residue was taken by suction filtration, 40 mL of dichloromethane was added to the filter residue, and the filter residue was taken by suction filtration to obtain the crude product of Intermediate 2 . White solid, yield 18%.
[0037] .
[0038] Two, the preparation of intermediate 3 ...
Embodiment 2
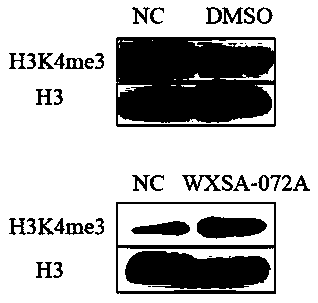
[0045] Example 2 Detecting the Effect of Compounds on RBP2 Enzyme Substrates in Gastric Cancer Cells (Western blot)
[0046] Purpose:
[0047] The effect of the test compound on the RBP2 enzyme substrate H3K4me3
[0048] Experimental Materials:
[0049] RPMI 1640 medium was purchased from Gibco;
[0050] Fetal bovine serum was purchased from Gibco;
[0051] DMSO was purchased from Sigma;
[0052] EpiQuik Total Histone Extraction Kit (OP-0006-100) was purchased from Epigentek Group;
[0053] Pierce ® BCA Protein Assay Kit was purchased from Thermo fisher scientific company
[0054] laboratory apparatus:
[0055] HF safe biological safety cabinet,
[0056] MCO-15AC carbon dioxide incubator, Japan Sanyo SANYO,
[0057] Polyacrylamide gel vertical electrophoresis tank, Liuyi Instrument Factory, Beijing, China
[0058] Test drug:
[0059] Compound WXSA-072A was prepared as a stock solution with DMSO, with a final concentration of 200mM, diluted with incomplete medium fo...
Embodiment 3
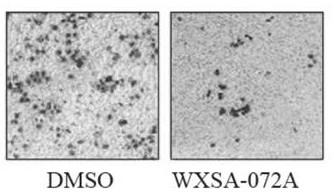
[0069] Example 3 Detection of compound's effect on migration ability in gastric cancer cells (Transwell)
[0070] Purpose:
[0071] The effect of detection compounds on the migration ability of gastric cancer cells
[0072] Experimental Materials:
[0073] RPMI 1640 medium was purchased from Gibco;
[0074] Fetal bovine serum was purchased from Gibco;
[0075] DMSO was purchased from Sigma;
[0076] Corning Transwell 3542 chamber was purchased from Beijing Suo Laibao Technology Co., Ltd.
[0077] laboratory apparatus:
[0078] HF safe biological safety cabinet,
[0079] MCO-15AC carbon dioxide incubator, Japan Sanyo SANYO,
[0080] Olympus CKX53 biological microscope, Shanghai Puhe Optoelectronics Technology Co., Ltd.,
[0081] Test drug:
[0082] For compound WXSA-072A, the sample was prepared into a mother solution with DMSO, with a final concentration of 200mM. When used, it was diluted with incomplete medium, and the final concentration of DMSO did not exceed 0.1%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com