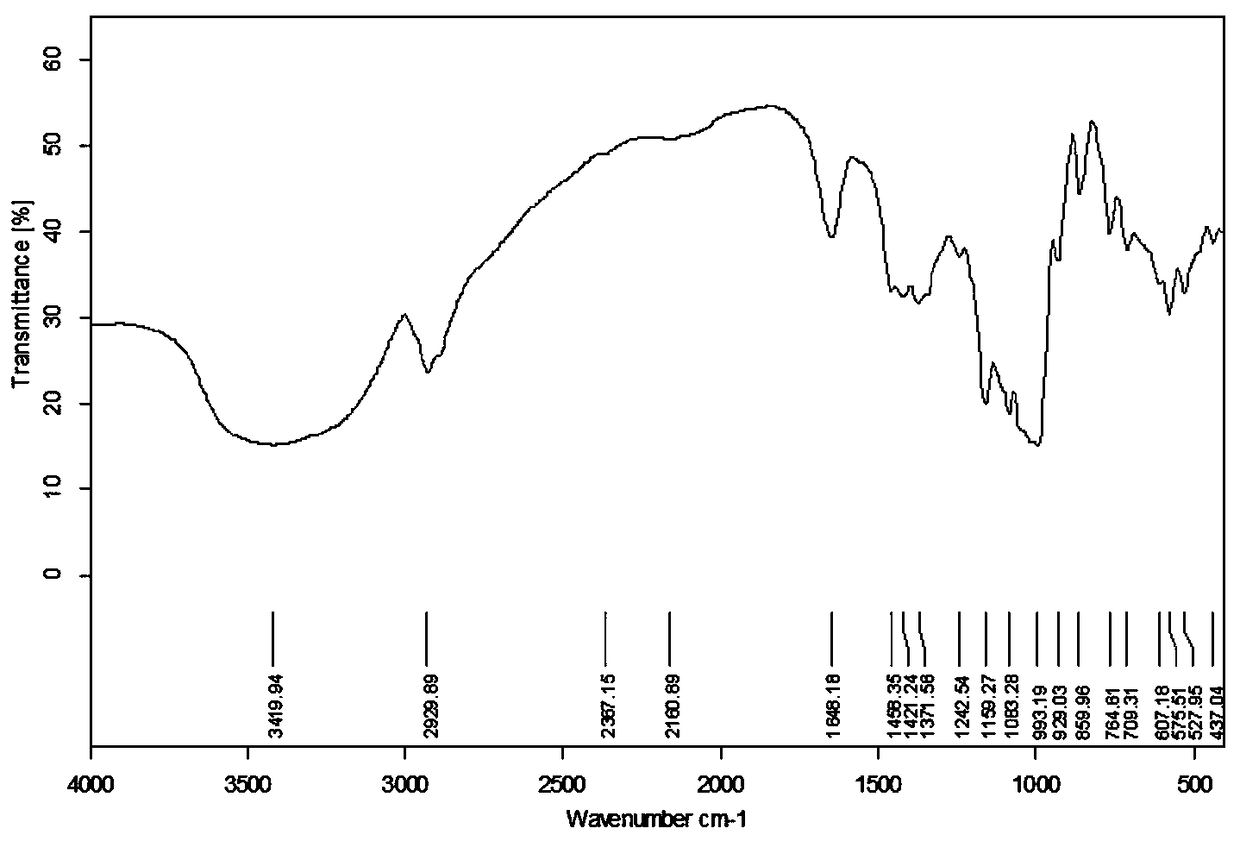
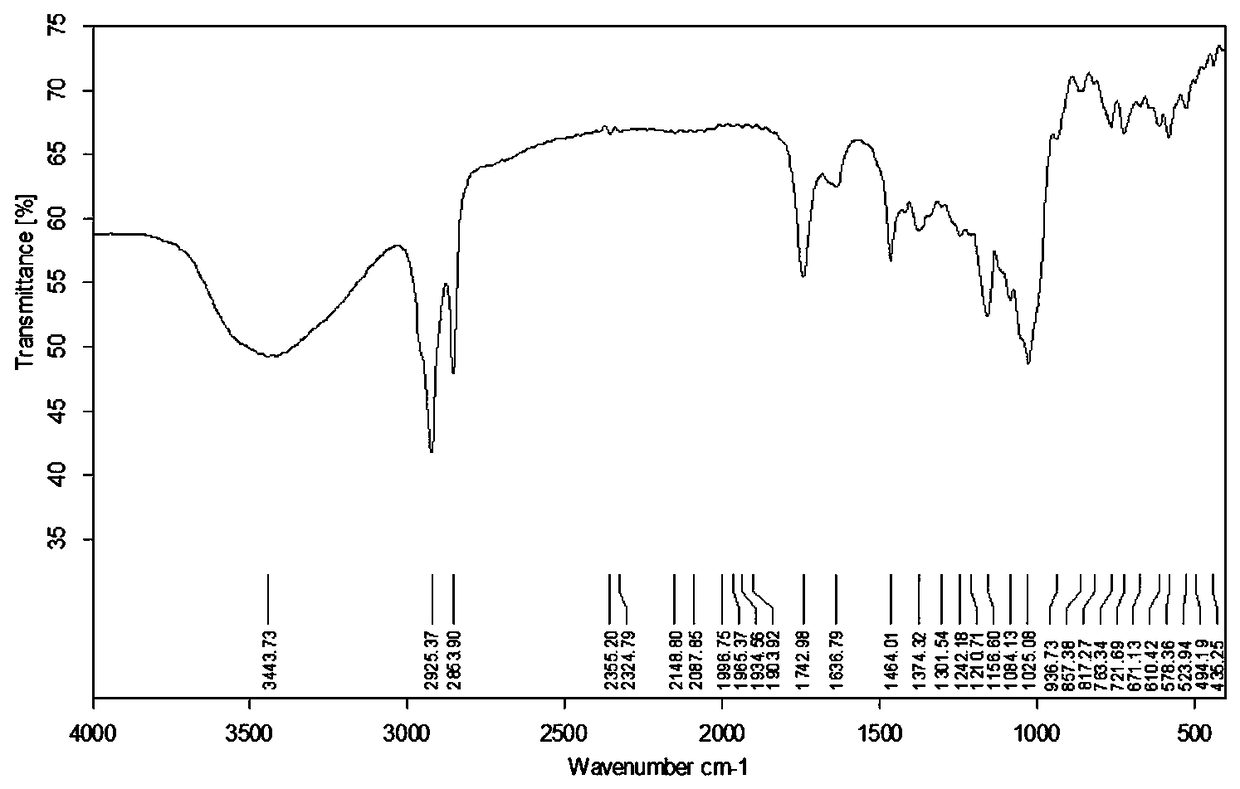
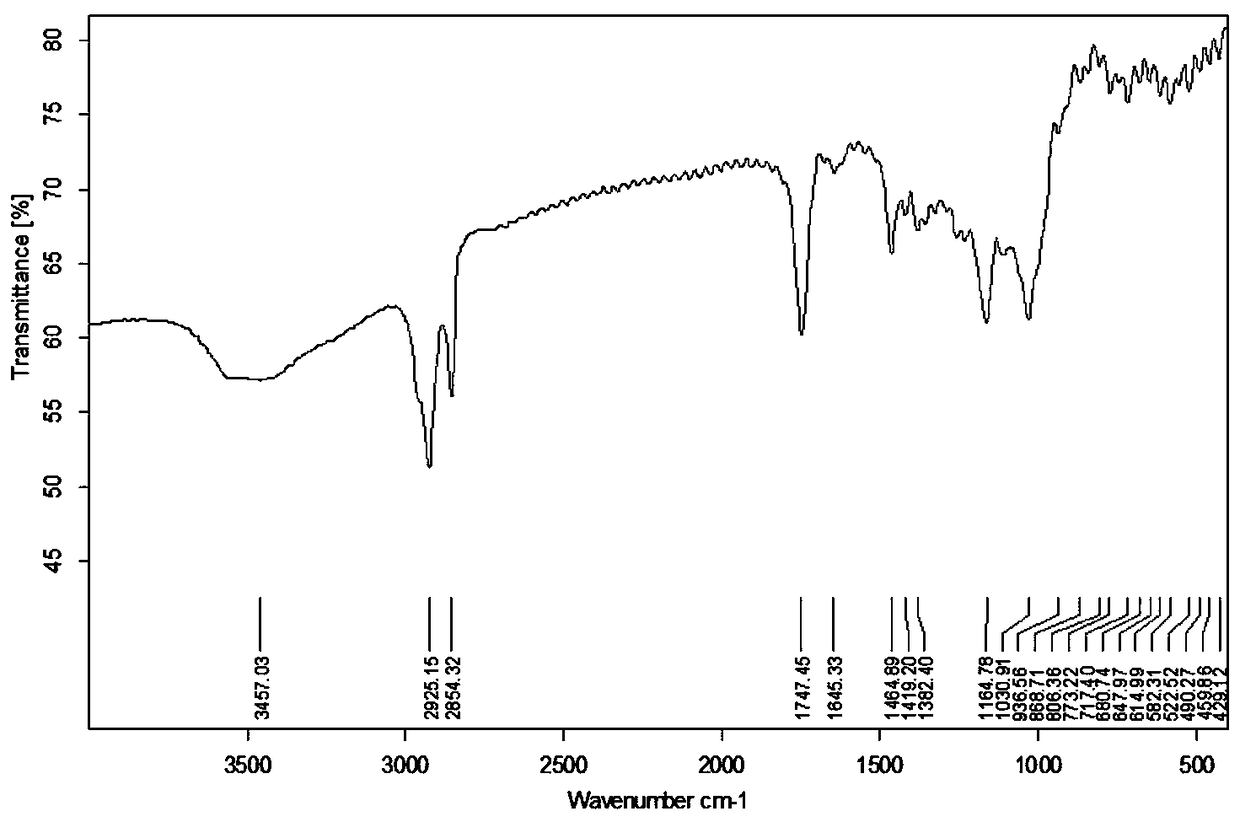
Preparation method and application of novel resistant starch with amylase inhibitory active group
An amylase inhibition and resistant starch technology is applied in the field of preparation of new resistant starch, can solve the problems of high fiber content, insufficient fiber content and little blood sugar regulation effect of resistant starch, and achieves the effect of high hypoglycemic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method for preparing a novel resistant starch with amylase inhibitory active groups, comprising the following steps:
[0028] (1) Add 0.1mol of palmitic acid into a 150mL three-necked flask, heat it in a water bath at 67°C until dissolved, add 0.16mol (11.6mL) of thionyl chloride dropwise under stirring, drop it in 30 minutes, continue stirring for 4 hours, stop the reaction, Recover the remaining thionyl chloride by distillation under reduced pressure to obtain palmitic acid chloride, which is poured into a dry 100 mL dropping funnel, mixed evenly, and sealed for later use;
[0029] (2) In a three-necked flask equipped with a stirrer and reflux condensed water, add 3.38g of potato cross-linked starch and 30mL of pyridine in sequence, stir to make them fully dispersed, activate the reaction at 95°C for 1 hour, adjust the reaction temperature to 85°C, Add 35mL of palmitic acid chloride dropwise, drop it for 30min, react for 2h, cool to room temperature, add the product...
Embodiment 2
[0033] A method for preparing a novel resistant starch with amylase inhibitory active groups, comprising the following steps:
[0034] (1) Add 0.1mol lauric acid into a 150mL three-necked flask, heat it in a water bath at 69°C until dissolved, add 0.16mol (11.6mL) thionyl chloride dropwise while stirring, and drop it in 35 minutes, continue stirring for 4.5 hours, and stop the reaction. The remaining thionyl chloride was recovered by distillation under reduced pressure to obtain lauric acid chloride, which was poured into a dry 100 mL dropping funnel, mixed evenly, and sealed for later use;
[0035] (2) In a three-necked flask equipped with a stirrer and reflux condensed water, add 3.38g of potato cross-linked starch and 30mL of pyridine in sequence, stir to make them fully dispersed, activate the reaction at 95°C for 1 hour, adjust the reaction temperature to 85°C, Add 35mL of lauric acid chloride dropwise, finish dropping after 30min, react for 2h, cool to room temperature, ...
Embodiment 3
[0038] A method for preparing a novel resistant starch with amylase inhibitory active groups, comprising the following steps:
[0039] (1) Add 0.1mol myristic acid to a 150mL three-necked flask, heat it in a water bath at 65°C until dissolved, add 0.16mol (11.6mL) thionyl chloride dropwise under stirring, drop it in 28min, continue stirring for 3.5h, stop the reaction , and recover the remaining thionyl chloride by distillation under reduced pressure to obtain myristic acid chloride, which is poured into a dry 100 mL dropping funnel, mixed evenly, and sealed for later use;
[0040] (2) In a three-necked flask equipped with a stirrer and reflux condensed water, add 3.38g of potato cross-linked starch and 30mL of pyridine in sequence, stir to make them fully dispersed, activate the reaction at 95°C for 1 hour, adjust the reaction temperature to 85°C, Add 35mL of myristic acid chloride dropwise, finish dropping in 30min, react for 2h, cool to room temperature, add the product int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com