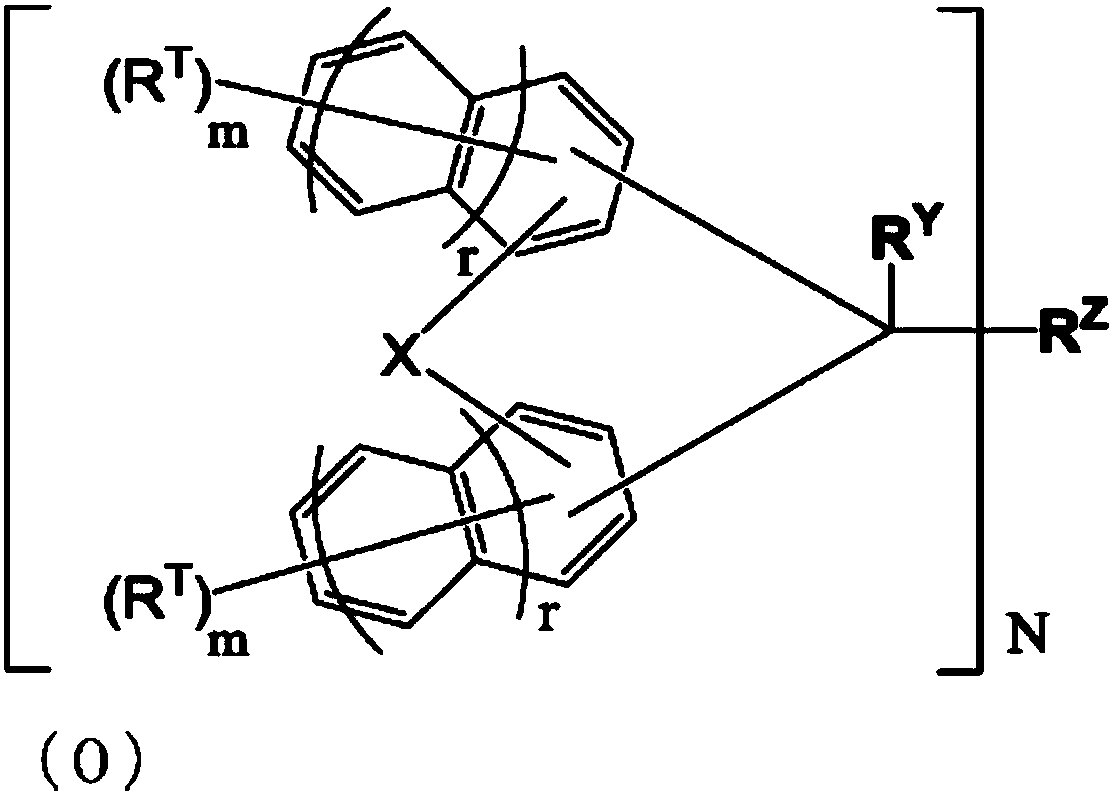
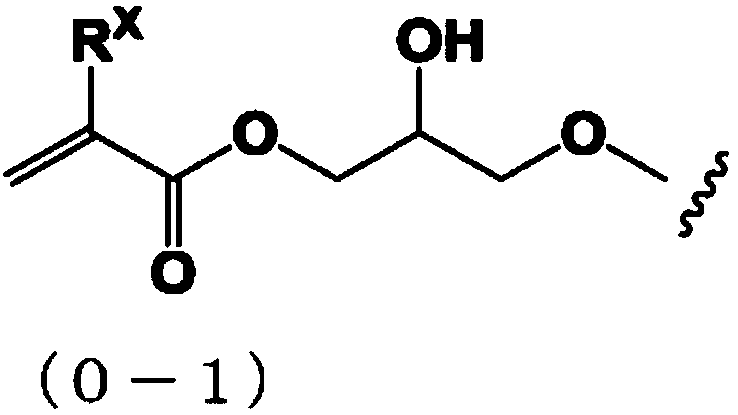
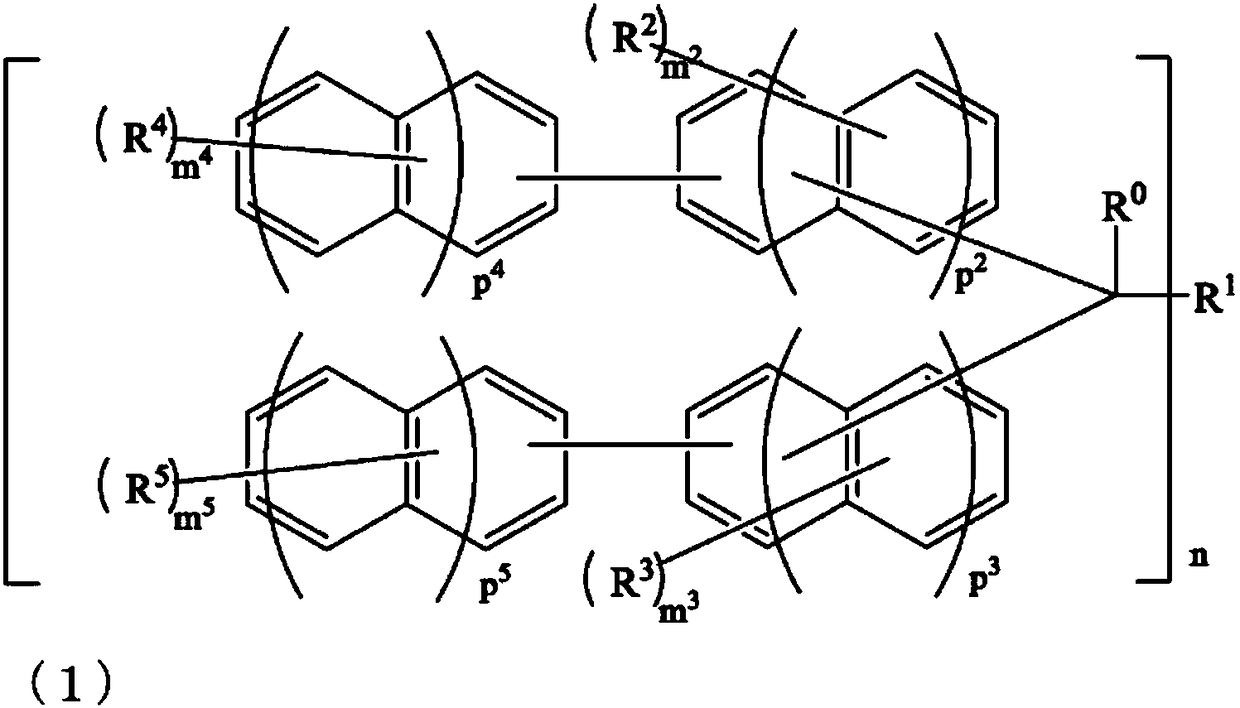
Compound, resin, composition, and pattern formation method
A compound and carbon number technology, applied in organic chemistry, photoengraving process of pattern surface, instruments, etc., can solve the problems of resist pattern collapse, resolution problem, difficulty in obtaining resist pattern, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0836] Hereinafter, this embodiment will be described in more detail based on synthesis examples and examples, but this embodiment is not limited to these examples.
[0837] (carbon concentration and oxygen concentration)
[0838] Carbon concentration and oxygen concentration (mass %) were measured by organic elemental analysis.
[0839] Device: CHN CORDER MT-6 (manufactured by Yanac Analytical Industry Co., Ltd.)
[0840] (molecular weight)
[0841] The molecular weight of the compound was measured by LC-MS analysis using Acquity UPLC / MALDI-Synapt HDMS manufactured by Water Corporation.
[0842] In addition, gel permeation chromatography (GPC) analysis was carried out under the following conditions to obtain polystyrene-equivalent weight average molecular weight (Mw), number average molecular weight (Mn), and degree of dispersion (Mw / Mn).
[0843] Device: Shodex GPC-101 (manufactured by Showa Denko Co., Ltd.)
[0844] Column: KF-80M×3
[0845] Eluent: THF 1mL / min
[084...
Synthetic example 1A
[0863] Synthesis of E-XBisN-1
[0864] In a container with an internal volume of 100ml equipped with a stirrer, a condensing tube and a burette, 10g (21mmol) of compound shown in the above formula (XBisN-1) and 14.8g (107mmol) of potassium carbonate are dropped into 50ml of dimethylformamide, and 6.56 g (54 mmol) of 2-chloroethyl acetate was stirred at 90° C. for 12 hours to react the reaction solution. Next, the reaction liquid was cooled in an ice bath to precipitate crystals, which were separated by filtration. Next, 40 g of the aforementioned crystals, 40 g of methanol, 100 g of THF and 24% aqueous sodium hydroxide solution were added to a container with an inner volume of 100 ml including a stirrer, a condenser, and a burette, and the reaction solution was stirred under reflux for 4 hours to react. Thereafter, it was cooled in an ice bath, the reaction solution was concentrated, and the precipitated solid was filtered and dried, followed by separation and purification b...
Synthetic example 2A
[0876] In the container of 100ml internal volume equipped with stirrer, condensing tube and burette, drop into the compound 11.2g (21mmol) shown in above-mentioned formula (BisF-1) and salt of wormwood 14.8g (107mmol) in 50ml dimethylformamide, 6.56 g (54 mmol) of 2-chloroethyl acetate was added, and the reaction solution was stirred at 90° C. for 12 hours to perform a reaction. Next, the reaction liquid was cooled in an ice bath to precipitate crystals, which were separated by filtration. Next, 40 g of the aforementioned crystals, 40 g of methanol, 100 g of THF and 24% aqueous sodium hydroxide solution were added to a container with an inner volume of 100 ml including a stirrer, a condenser, and a burette, and the reaction solution was stirred under reflux for 4 hours to react. Afterwards, it was cooled in an ice bath, the reaction solution was concentrated, and the precipitated solid was filtered and dried, followed by separation and purification by column chromatography to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com