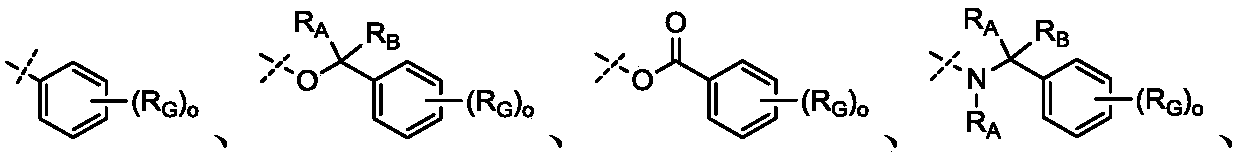
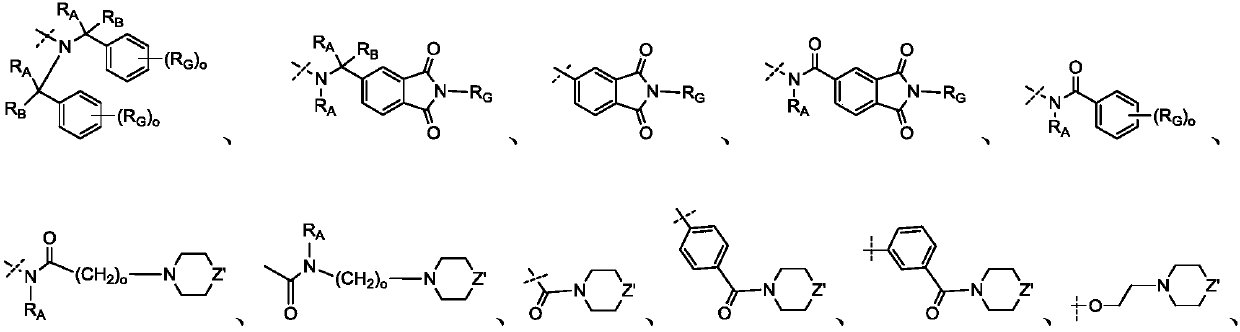
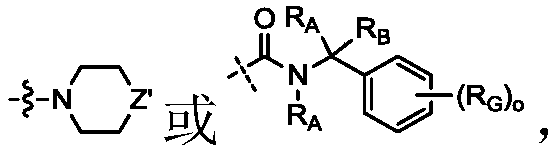
Novel cyclosporin derivatives and uses thereof
A halogen, compound technology, applied in the field of novel cyclosporine derivatives, can solve problems such as cell death and mitochondrial swelling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0370] [α-Methylene-Sar]-3-cyclosporine
[0371]
[0372] To a solution of [α-hydroxymethyl-Sar]-3-cyclosporin (246mg, 0.20mmol) in THF (15ml) was added sodium hydride (120mg, 60% in mineral oil, 3mmol) and dichlorophosphoric acid Ethyl ester (412 mg, 2.40 mmol). The resulting mixture was stirred overnight at room temperature. After disappearance of starting material, methanol (10 mL) and potassium tert-butoxide (33 mg, 26.00 mmol) were added. The mixture was stirred at room temperature for 3 hours, and the solvent was removed under reduced pressure. The residue was dissolved in dichloromethane (50ml). The dichloromethane layer was washed with brine, dried over magnesium sulfate and evaporated under reduced pressure. Purification of the residue by flash chromatography using dichloromethane / methanol as eluent afforded 205 mg of the product [molecular formula: C 63 h 111 N 11 o 12 ; Exact mass: 1213.84; MS (m / z): 1214.59 (M+1) + ; HPLC RT: 17.47 min. (C8 reverse-phas...
Embodiment 2
[0374] [(3R,4R)-3-Hydroxy-4-methyl-6-(4-methoxycarbonylbenzyl)-N-MeNle]-1-[(S)-(4-Hydroxybutyl Thio)methyl-Sar]-3-cyclosporine
[0375] [3-Acetyl-MeBmt]-1-cyclosporin
[0376]
[0377] To a dry flask under nitrogen was added cyclosporine (12.00 g, 9.98 mmol), N,N-dimethylaminopyridine (0.12 g, 0.10 mmol), anhydrous pyridine (120 ml) and acetic anhydride (54 ml, 0.54mol). After stirring overnight at room temperature, the mixture was poured into ice water (600ml) and stirred until the ice melted. Ethyl acetate (100ml) was added, and the mixture was separated. The ethyl acetate layer was washed with 1.0N hydrochloric acid solution (100ml x 2), saturated sodium bicarbonate solution (100ml), brine (100ml), dried over magnesium sulfate and evaporated under reduced pressure. The residue was purified by chromatography (hexane / acetone) to obtain 11.40 g of pure [3-acetyl-MeBmt]-1-cyclosporine [molecular formula: C 64 h 113 N 11 o 13 ; Exact mass: 1243.85; MS (m / z): 1244.5...
Embodiment 3
[0400] [(3R,4R)-3-Hydroxy-4-methyl-6-(4-carboxybenzyl)-N-MeNle]-1-[(S)-(4-hydroxybutylthio) Methyl-Sar]-3-cyclosporine
[0401]
[0402] To [(3R,4R)-3-hydroxy-4-methyl-6-(4-methoxycarbonylbenzyl)-N-MeNle]-1-[(S)-(4-hydroxybutylthio) To a solution of methyl-Sar]-3-cyclosporin (85mg, 0.06mmol) in methanol (5ml) was added a solution of lithium hydroxide (15mg) in water (5ml). The reaction mixture was stirred overnight at room temperature. Most of the methanol was evaporated under reduced pressure. The pH of the mixture was adjusted to 6 with 1.0N hydrochloric acid. Ethyl acetate (60ml) and brine (10ml) were added, and the mixture was separated. The organic layer was dried over magnesium sulfate and evaporated under reduced pressure. Purification of the residue afforded pure [(3R,4R)-3-hydroxy-4-methyl-6-(4-carboxybenzyl)-N-MeNle]-1-[(S)-(4-hydroxybutyl Thio)methyl-Sar]-3-cyclosporine [molecular formula: C 73 h 125 N 11 o 15 S; Exact mass: 1427.91; MS (m / z): 1428.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com