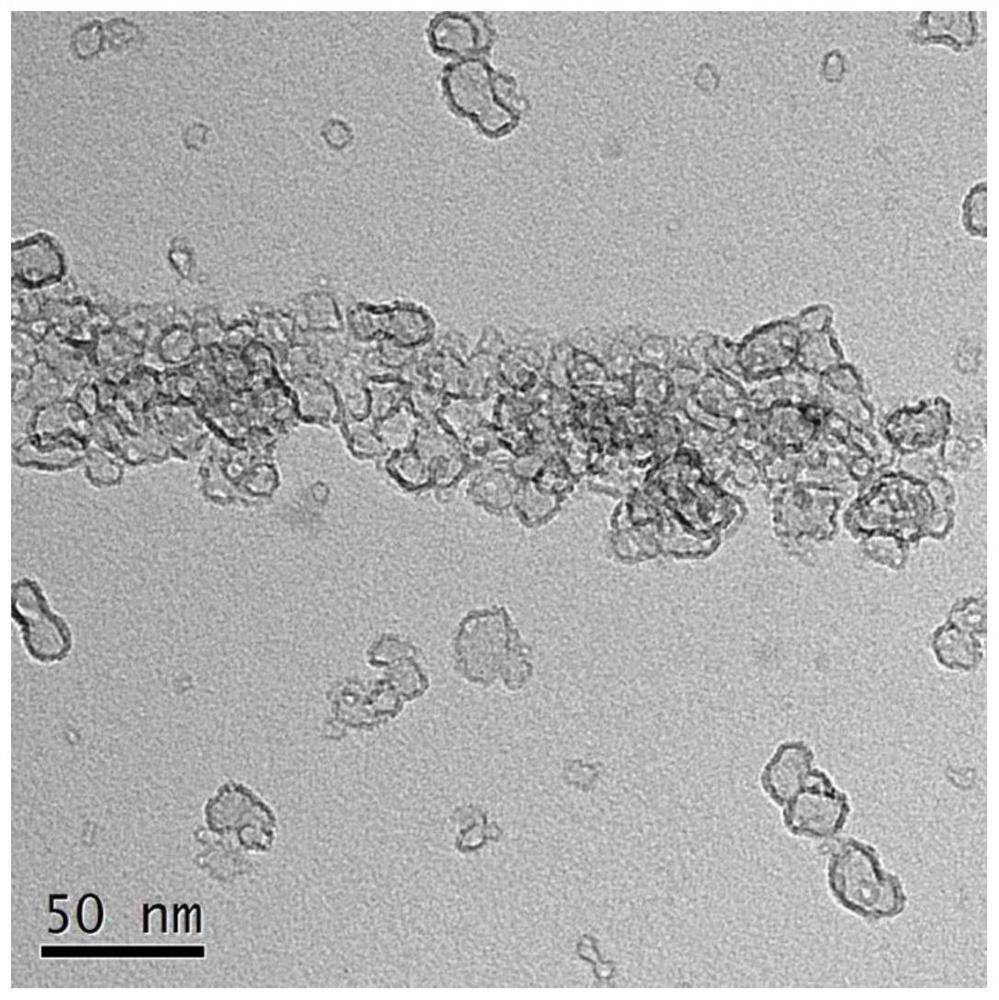
Chromium-free and environment-friendly catalyst for synthesis of 1,1,1,3,3,3-hexafluoro-2-butene by gas-phase fluorination
A chemical synthesis and catalyst technology, which is applied in physical/chemical process catalysts, catalyst activation/preparation, organic chemistry, etc., can solve problems such as low catalyst activity, human health threats, ecological environment pollution, etc., and achieve large specific surface area and anti-sintering Superior performance, good anti-sintering ability at high temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: prepare M / MgF 2 catalyst
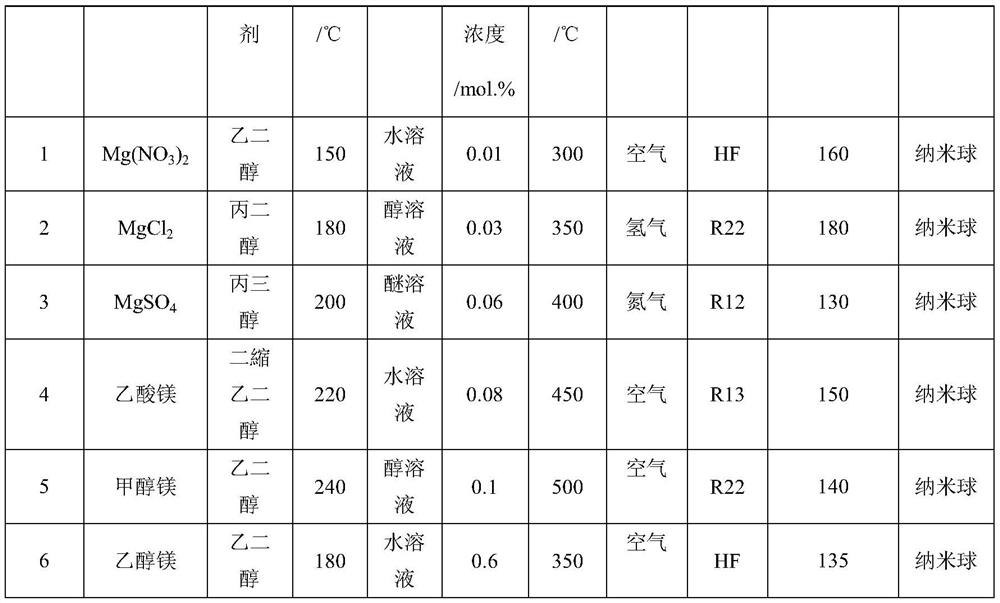
[0028] Dissolve 1.0M magnesium source and 0.01-0.1M modified metal ions in 50mL polyol solvent, add the fluorinating reagent dropwise to the above solution under stirring at 150-240°C, the dropping time is 15min, after the dropping is completed Stir again for 6-12h to obtain M / MgF 2 Dry gel; then roast in an air atmosphere or a hydrogen atmosphere at 300-500°C for more than 5 hours, and finally perform a fluorination treatment on the roasted material at a temperature of 150-400°C to obtain a catalyst. The fluorine reagents are gaseous hydrogen fluoride and dichlorodifluoromethane , one chlorodifluoromethane, one chlorotrifluoromethane any one. The textural properties of magnesium fluoride prepared under different magnesium sources, polyol solvents, coagulants, fluorinating reagents, and calcination temperatures are shown in Table 1.
[0029] The physicochemical property result of the magnesium fluoride-based catalyst of table ...
Embodiment 2
[0033] Adopt the same method to prepare M / MgF in embodiment 1 2 catalyst, which was applied to the gas-phase fluorination of CF 3 CHClCH 2 CCl 3 In the reaction of synthesizing HFO-1336, after running for 12 hours, the reaction results are as follows:
[0034] The reaction evaluation result of the magnesium fluoride-based catalyst of table 2 embodiment 2
[0035]
Embodiment 3
[0037] Adopt same method to prepare M / MgF in embodiment 1 2 catalyst, which was applied to the gas-phase fluorination of CF 3 CHClCH 2 CCl 3 In the reaction of synthesizing HFO-1336, after running for 12 hours, the reaction results are as follows:
[0038] The reaction evaluation result of the magnesium fluoride-based catalyst of table 3 embodiment 3
[0039]
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com