Method for preparing 2,5-dimethylfuran through hydrogenation of 5-hydroxymethyl furfural
A technology for hydroxymethylfurfural and dimethylfuran, which is applied in the field of preparation of 2,5-dimethylfuran by catalyzing the hydrogenation of 5-hydroxymethylfurfural to achieve the effects of excellent yield, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] Preparation example of HCP-Co nanosheets
[0030] (1) Dissolve cobalt nitrate hexahydrate in deionized water to prepare mixed solution A, then add 2mol / L sodium hydroxide solution dropwise to mixed solution A to obtain suspension B, then carry out suspension B Suction filtration and washing with water for 3 to 5 times to obtain the precipitate, and place the precipitate in a vacuum drying oven at 60°C to dry overnight to obtain the precursor β-Co(OH) 2 ,
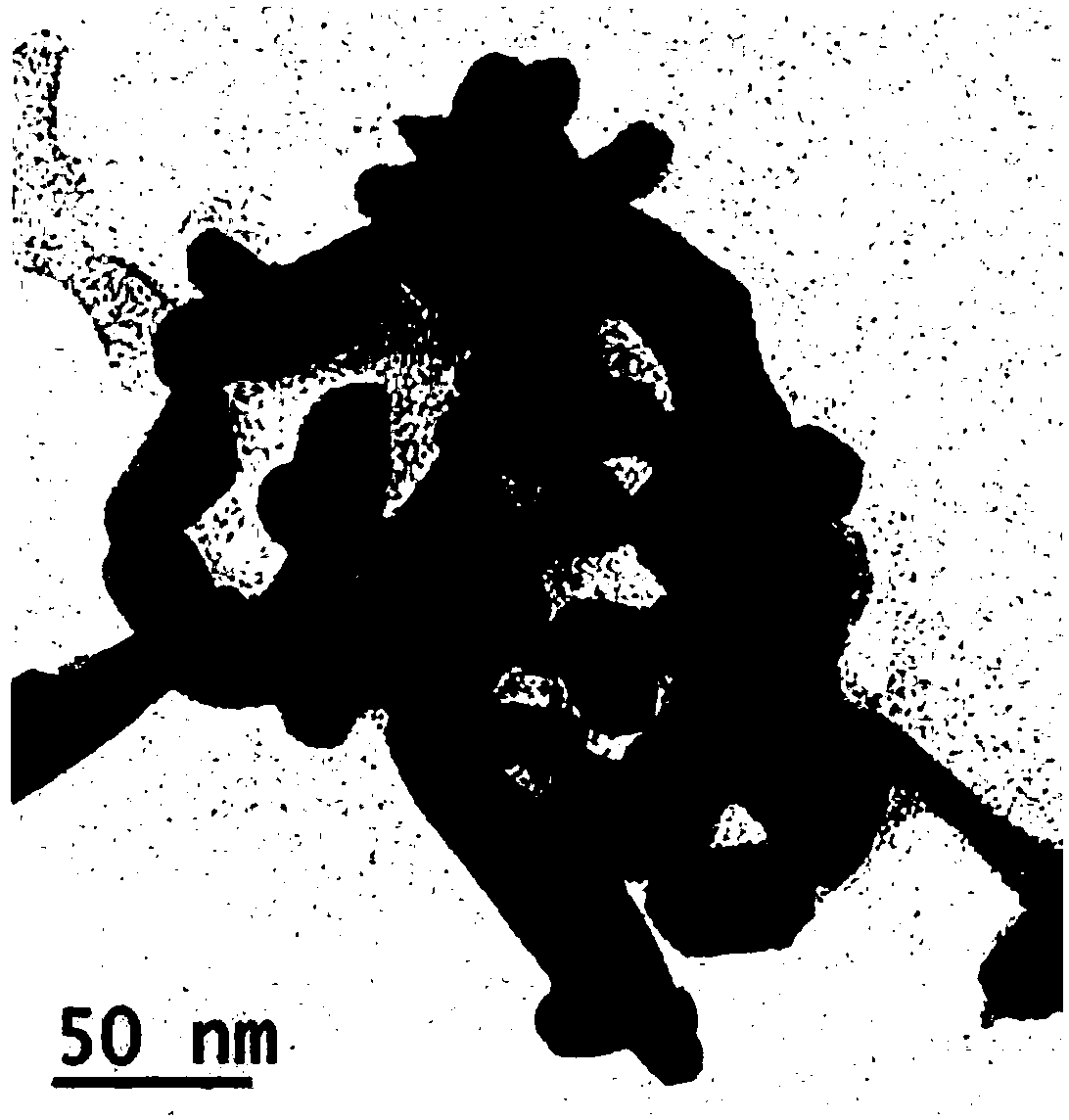
[0031] (2) The precursor β-Co(OH) 2 The nanosheets are placed in a quartz tube, and in an atmosphere of argon-hydrogen (9:1) flow rate of 40-80mL / min, the pressure is maintained at 1-1.2bar, and the temperature is raised to 320°C at a heating rate of 9-13°C / min , keep the temperature for 2 ~ 3h, and then naturally cool to room temperature under the atmosphere, then passivate in the nitrogen-oxygen (9:1) atmosphere to obtain HCP-Co mesoporous nanosheets, the obtained HCP-Co mesoporous nanosheets The average size is ...
Embodiment 1
[0037] The hydrogenation of 5-hydroxymethylfurfural to prepare 2,5-dimethylfuran includes the following steps: (1) before the reaction, fill the autoclave with 1.0MPa hydrogen, exhaust the gas 5 times, and then fill it with 1.6MPa hydrogen ;
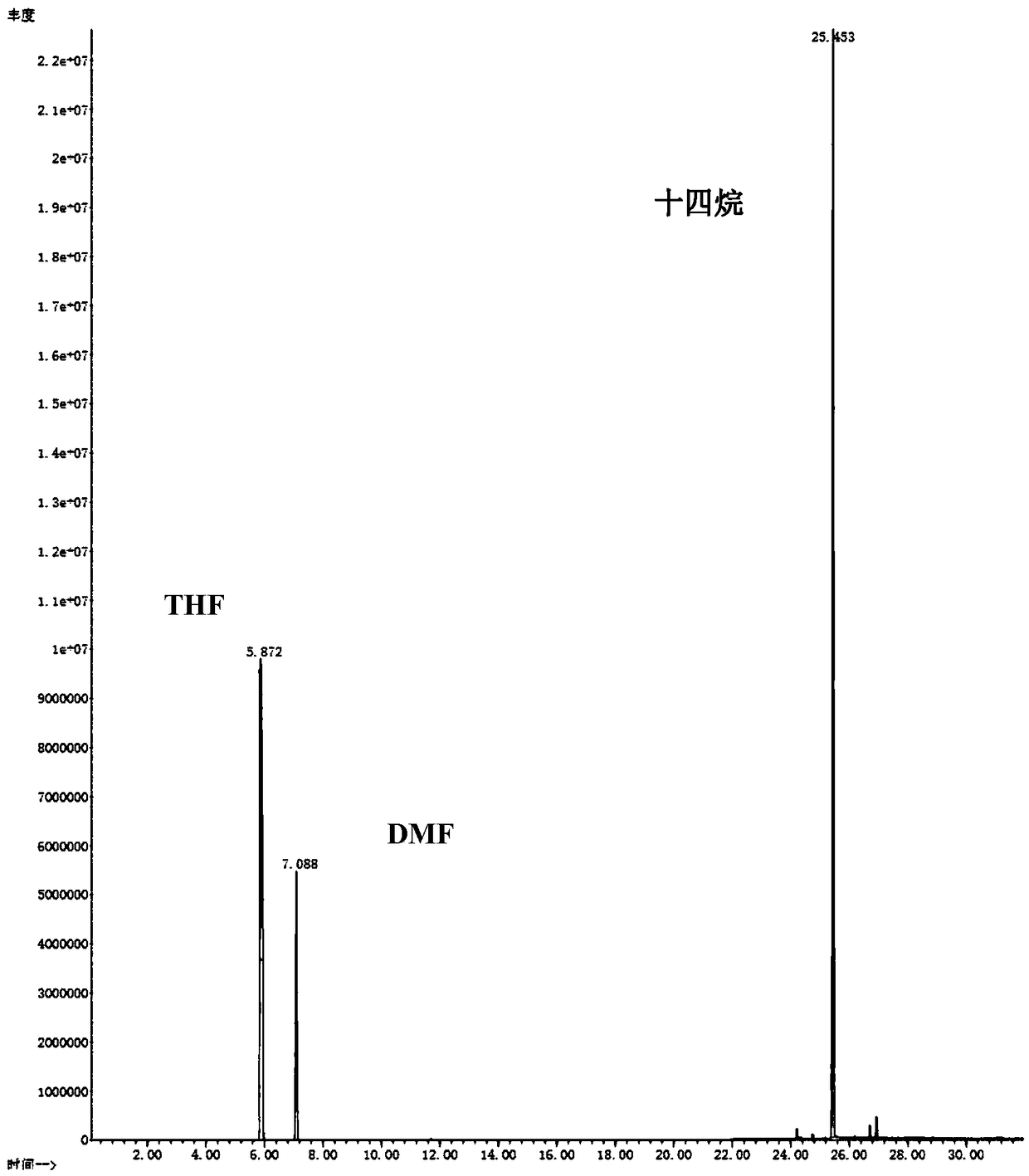
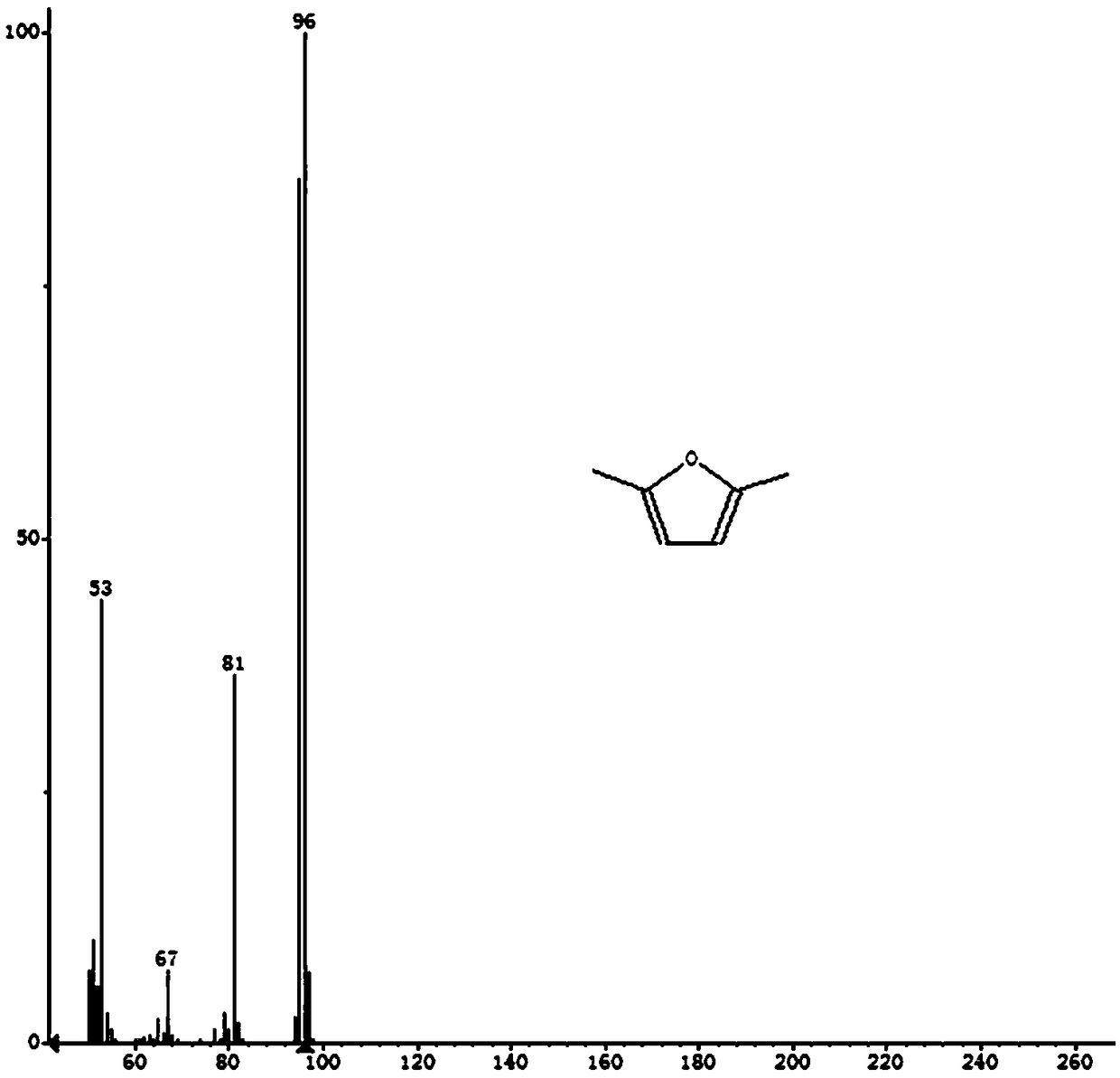
[0038] (2) Put 0.05g HCP-Co as a catalyst and 12ml tetrahydrofuran solvent into the autoclave, then add 1.0mmol 5-hydroxymethylfurfural, and feed 1.6Mpa of H 2, react at 180°C for 4 hours, and maintain a stirring speed of 500r / min during the reaction to obtain 2,5-dimethylfuran. After the reaction, take down the reactor and cool it down to room temperature naturally. The products were collected and analyzed by Agilent 7890B gas chromatography. The types of products were identified by GC-MS model 7890B-5977B, showing that the conversion rate of HMF was 93.9%, and the yield of DMF reached 38.9%.
[0039] It shows that the hydrogenation reaction of HMF is violent, and HMF reacts with hydrogen. The reason for the reaction is that HCP-Co act...
Embodiment 2
[0041] 5-Hydroxymethylfurfural is hydrogenated to prepare 2,5-dimethylfuran, including the following steps: (1) Before the reaction, fill the autoclave with 1.0MPa hydrogen, exhaust the gas 5 times, and then fill it with 1.8MPa hydrogen ;
[0042] (2) Put 0.05g HCP-Co as a catalyst and 12ml tetrahydrofuran solvent into the autoclave, then add 1.0mmol 5-hydroxymethylfurfural, and feed 1.8Mpa of H 2 , react at 180°C for 4 hours, and maintain a stirring speed of 500r / min during the reaction to obtain 2,5-dimethylfuran. After the reaction, take down the reactor and cool it down to room temperature naturally. The products were collected and analyzed by Agilent 7890B gas chromatography. The types of products were identified by GC-MS model 7890B-5977B, showing that the conversion rate of HMF was 98.7%, and the yield of DMF reached 94.9%.
[0043] It shows that the hydrogenation reaction of HMF is violent, and HMF reacts with hydrogen. The reason for the reaction is that HCP-Co plays...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com