Anti-mullerian hormone magnetic particle chemiluminescence detection kit
A chemiluminescence reagent, Müllerian hormone technology, applied in chemiluminescence/bioluminescence, analysis by chemical reaction of materials, etc., can solve the problem of RIA method pollution, gas chromatography and mass spectrometry combined method is not suitable for clinical detection , ELISA method has low degree of automation and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] A preparation method of a magnetic particle chemiluminescence detection kit for measuring the content of human anti-Müllerian hormone (AMH), comprising the steps of:
[0059] Preparation of reagent R1: 1) Prepare buffer according to the content of reagent R1 buffer components, adjust pH; 2) Prepare fluorescein isothiocyanate-labeled anti-Müllerian hormone (AMH) monoclonal antibody; 3) Mix isothiocyanate Fluorescein cyanate-labeled anti-Müllerian hormone (AMH) monoclonal antibody was diluted with R1 buffer.
[0060] Preparation of reagent R2: 1) Prepare buffer according to the content of reagent R2 buffer components, and adjust pH; 2) Prepare alkaline phosphatase-labeled anti-Müllerian hormone (AMH) antibody; 3) Alkaline phosphatase-labeled Anti-Müllerian hormone (AMH) antibody was diluted with R2 buffer.
[0061] Preparation of magnetic separation reagents: 1) preparation of buffer solution according to the content of magnetic particle buffer components; 2) preparation...
Embodiment 1
[0072] Example 1 A magnetic particle chemiluminescence detection kit for determining the content of human anti-Müllerian hormone (AMH) includes R1 reagent, R2 reagent, magnetic separation reagent, calibrator solution series and substrate solution.
[0073] In this embodiment, the R1 reagents include: 1) R1 antibody: fluorescein isothiocyanate (FITC)-labeled anti-Müllerian hormone (AMH) monoclonal antibody at a concentration of 1.0-3.0 μg / mL; 2) buffer Solution: including sodium azide, the concentration is 0.989g / L; bovine serum albumin, the concentration is 4.5g / L; trishydroxymethyl aminomethane, the molar concentration is 0.05mol / L; the rest is deionized water. The buffer pH of the R1 reagent is preferably 7.0.
[0074] In this embodiment, the R2 reagent includes: 1) R2 antibody: alkaline phosphatase-labeled anti-Müllerian hormone (AMH) antibody at a concentration of 1.5 μg / ml; 2) buffer solution: including sodium azide at a concentration of 0.989g / L; bovine serum albumin, t...
Embodiment 2
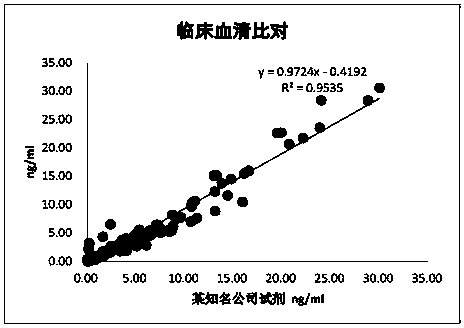
[0078] Example 2 Performance evaluation of the anti-Müllerian hormone (AMH) magnetic particle chemiluminescent detection kit of the present invention
[0079] 1. Minimum detection limit
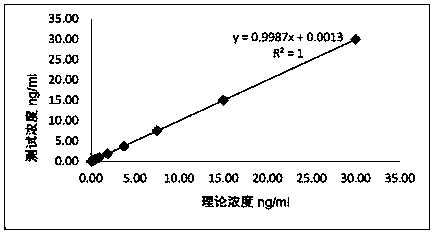
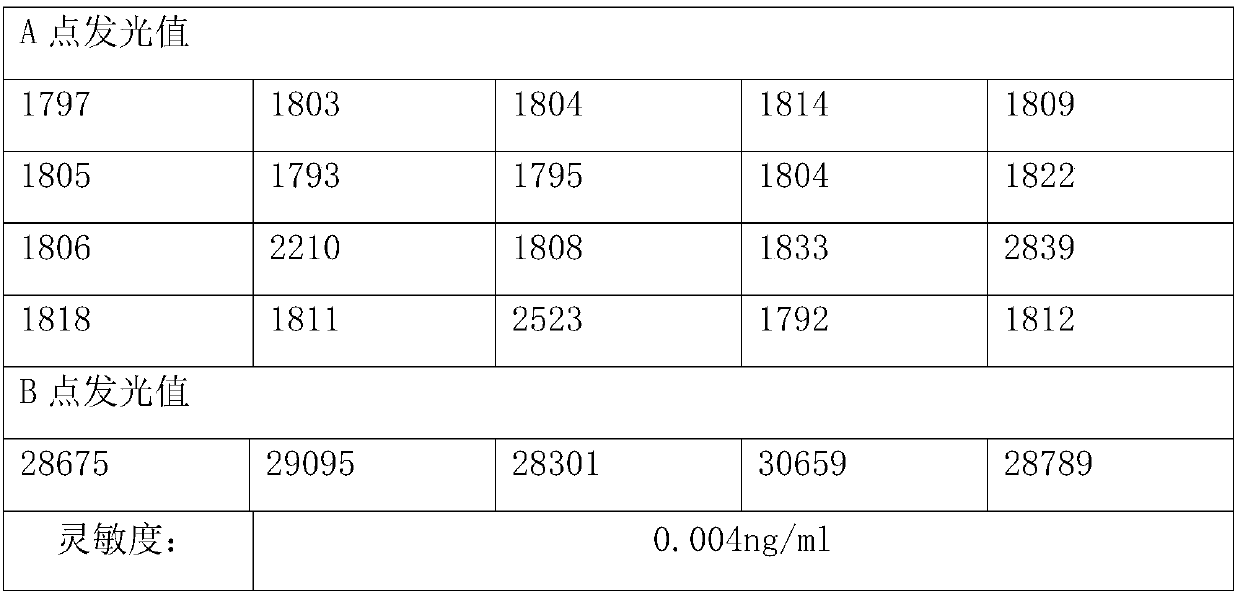
[0080] Method: Use the zero-concentration calibrator as a sample for detection, repeat the measurement 20 times, obtain the relative luminous intensity (RLU) value of the 20 measurement results, calculate the mean (M) and standard deviation (SD), and obtain M+ 2SD, according to the concentration-RLU between the zero concentration calibrator and the adjacent calibrator, a two-point regression fitting is performed to obtain a linear equation, and the RLU value of M+2SD (positive reaction) or M-2SD (negative reaction) is brought into In the above equation, the corresponding concentration value is obtained, which is the minimum detection limit.
[0081] Qualified standard: <0.05ng / ml
[0082]
[0083] Conclusion: The sensitivity is not greater than 0.05ng / mL, meeting the design requirements....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com