Gas chromatographic-mass spectrometric detection method of higenamine in multiple matrix samples
A technique for higenamine and mass spectrometry detection, applied in the field of book chemical analysis, can solve the problems such as the detection limit does not meet the ideal requirements, there is no analysis and research on Chinese patent medicines, and it does not have universal applicability, etc., and the extraction method is easy to operate. , the effect of improving sensitivity and accuracy, and low operating cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 sample: blood plasma
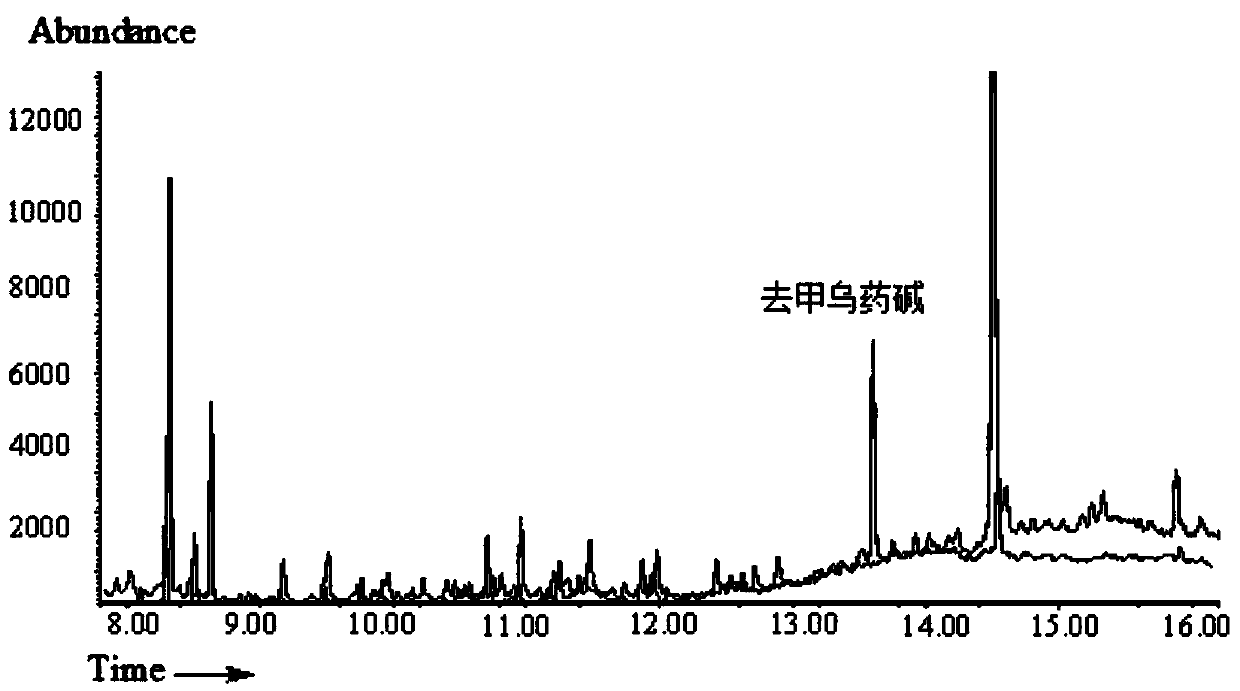
[0034] After the plasma sample was thawed, the protein was precipitated with acetonitrile. The volume ratio of plasma to sample was 1:3. After precipitation, the supernatant was taken as the sample solution. Use PEP-2 (200mg / 6mL) solid-phase extraction column for extraction, first use 5mL methanol and 5mL water to activate the solid-phase extraction (SPE) column. Take 1mL sample solution for solid phase extraction, then rinse with 1mL 30% (V) methanol aqueous solution, and finally elute with 2mL 5% (V) formic acid methanol solution, and dry the eluent slowly with nitrogen flow at 70°C. Add 100 μL of heptafluorobutyric anhydride to the dried residue and derivatize at 70°C for 45 min. The derivatized sample solution is detected by gas chromatography-mass spectrometry, and the chromatogram is shown in figure 1 . figure 1 The curves in the middle are the curves of the blank plasma sample and the plasma sample after adding the standard sam...
Embodiment 2
[0035] Example 2: Sample: Urine
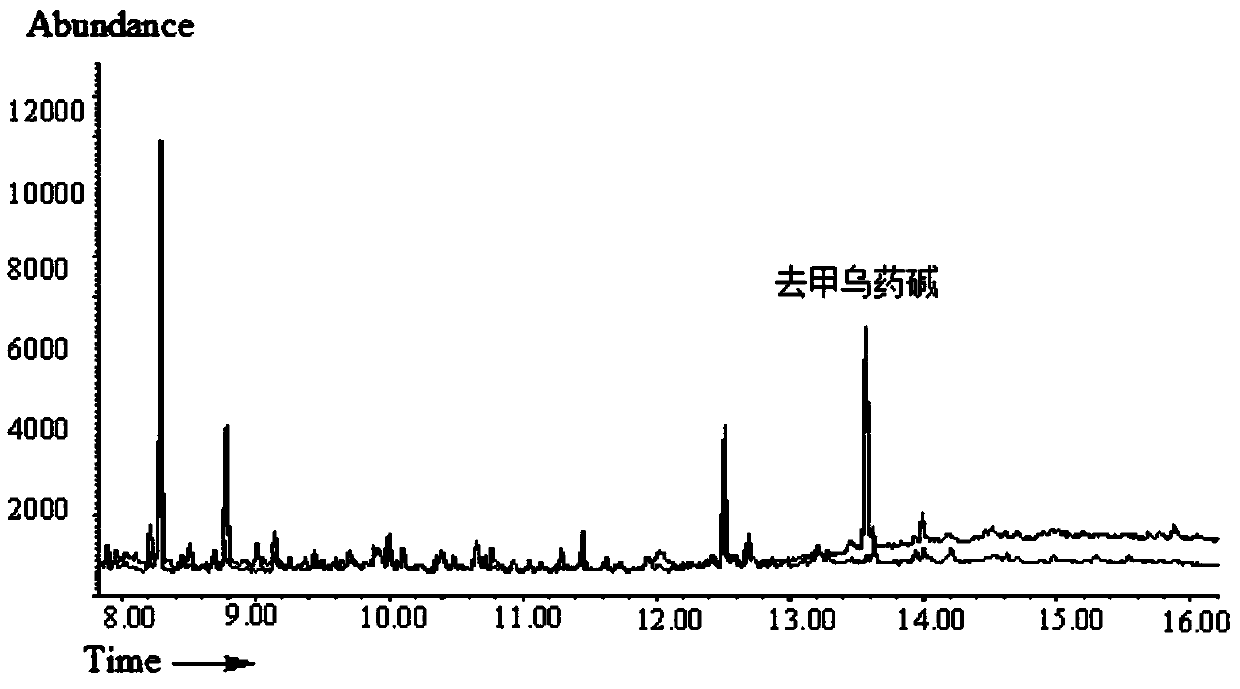
[0036]After the urine sample was thawed, the protein was precipitated with acetonitrile. The volume ratio of urine to sample was 1:3. After precipitation, the supernatant was taken as the sample solution. Take the supernatant as the sample solution. Use PEP-2 (200mg / 6mL) solid-phase extraction column for extraction, first use 5mL methanol and 5mL water to activate the solid-phase extraction (SPE) column. Take 1mL sample solution for solid phase extraction, then rinse with 1mL 30% (V) methanol aqueous solution, and finally use 2mL 5% (V) formic acid methanol solution for elution, and dry the eluent slowly with nitrogen flow at 70°C . Add 100 μL of heptafluorobutyric anhydride to the dried residue and derivatize at 70°C for 45 min. The derivatized sample solution is detected by gas chromatography-mass spectrometry, and the chromatogram is shown in figure 2 . figure 2 The middle curves are the blank urine sample and the urine sample after ...
Embodiment 3
[0037] Embodiment 3: sample solution preparation Chinese patent medicine
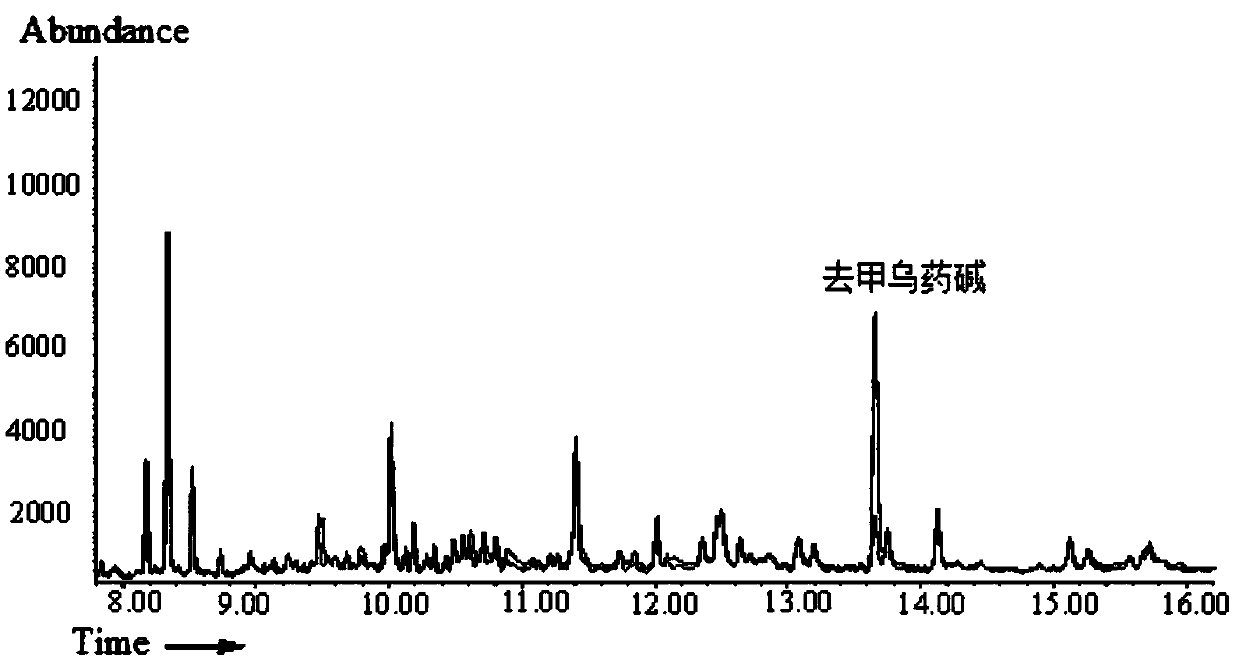
[0038] Take 50 μL of the solution and dilute it with water to 1 mL. Use PEP-2 (200mg / 6mL) solid-phase extraction column for extraction, first use 5mL methanol and 5mL water to activate the solid-phase extraction (SPE) column. Take 1mL sample solution for solid phase extraction, then rinse with 1mL 30% (V) methanol aqueous solution, and finally use 2mL 5% (V) formic acid methanol solution for elution, and dry the eluent slowly with nitrogen flow at 70°C . Add 100 μL of heptafluorobutyric anhydride to the dried residue and derivatize at 70°C for 45 min. The derivatized sample solution is detected by gas chromatography-mass spectrometry, and the chromatogram is shown in image 3 . image 3 The curves in the middle are the Chinese patent medicine samples in the blank solution and the Chinese patent medicine samples in the solution after adding the standard sample (25ng / mL). After being processed by the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| linear range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com