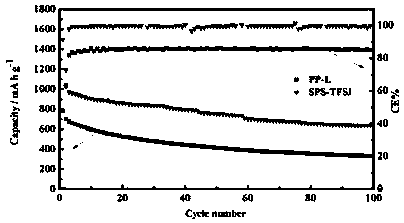
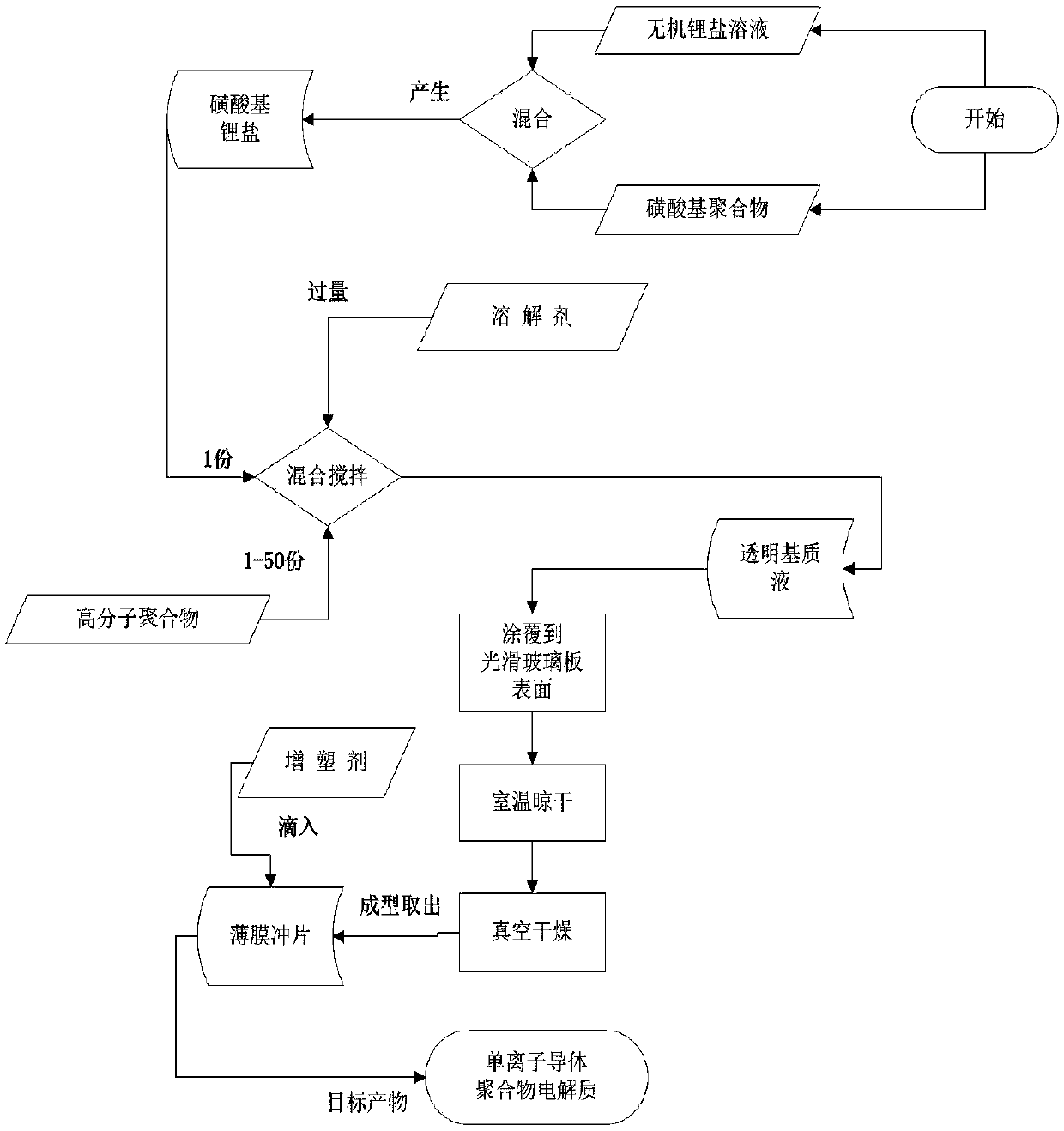
Method for preparing polymer electrolyte for improving self-discharge of lithium-sulfur battery and application
A lithium-sulfur battery and electrolyte technology, which is applied in the preparation and application of single-ion conductor polymer electrolytes, can solve the problems that affect the stability of lithium negative electrodes, the electrochemical stability of batteries, the large pores of polyolefin diaphragms, and the flammability of liquid electrolytes. Achieve the effect of avoiding self-discharge phenomenon, reducing battery cost, and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Polymer electrolyte material and preparation method of sulfonated polystyrene lithium and polyvinylidene fluoride-hexafluoropropylene copolymer. The specific raw materials and preparation process of the single ion conductor polymer electrolyte are as follows:
[0040] (1) sulfonated polystyrene and lithium hydroxide solution are mixed, react until sulfonated polystyrene lithium;
[0041] (2) prepare a certain amount of N,N-dimethylformamide;
[0042] (3) Take the prepared sulfonated polystyrene lithium and polyvinylidene fluoride-hexafluoropropylene copolymer according to the molar ratio of 1:10; then the sulfonated polystyrene lithium and polyvinylidene fluoride-hexafluoropropylene The copolymer is added to a certain amount of N,N-dimethylformamide, stirred and dissolved to obtain a uniform transparent solution;
[0043] (4) Use a scraper to pour a transparent solution on the surface of a clean glass plate, then apply it with a scraper, place it at room temperature f...
Embodiment 2
[0047] Polymer electrolyte material and preparation method of lithium polyalkylmethacrylate sulfonate and polyvinylidene fluoride. The specific raw materials and preparation process of the single ion conductor polymer electrolyte are as follows:
[0048] (1) polyalkylmethacrylate sulfonic acid and lithium hydroxide solution are mixed, and reaction obtains polyalkylmethacrylate sulfonate lithium;
[0049] (2) prepare a certain amount of N,N-dimethylacetamide;
[0050] (3) Take the prepared lithium polyalkyl methacrylate sulfonate and polyvinylidene fluoride according to a molar ratio of 1:20; then add lithium polyalkyl methacrylate sulfonate and polyvinylidene fluoride to a certain amount Amount of N,N-dimethylacetamide, stirred and dissolved to obtain a uniform transparent solution;
[0051] (4) Spread the transparent solution on a smooth glass plate by pouring, and then carry out the process of placing it at room temperature for 24 hours to dry naturally, and then drying it...
Embodiment 3
[0055] Polymer electrolyte material and preparation method of hyperbranched lithium polyurethane containing sulfonic acid groups and polyethylene oxide. The specific raw materials and preparation process of the single ion conductor polymer electrolyte are as follows:
[0056] (1) the hyperbranched polyurethane containing sulfonic acid group is mixed with lithium sulfate solution, and the reaction obtains the hyperbranched polyurethane lithium containing sulfonic acid group;
[0057] (2) prepare a certain amount of N-methylpyrrolidone amine;
[0058] (3) Take the prepared hyperbranched polyurethane lithium and polyethylene oxide containing sulfonic acid groups according to a molar ratio of 1:30; then add a certain amount of hyperbranched polyurethane lithium and polyethylene oxide containing sulfonic acid groups Amount of N-methylpyrrolidone amine, stirred and dissolved to obtain a uniform transparent solution;
[0059] (4) Using the micro-concave transfer coating process to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com