Triple inactivated vaccine used for porcine circovirus-3, porcine parvovirus and swine influenza and preparation method thereof
A technology for porcine circovirus and parvovirus, applied in biochemical equipment and methods, vaccines, viruses, etc., can solve the problems of high cost, poor prevention and control effect, and large immunization workload, and achieve high cost and convenient immunization. , the effect of high titer content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
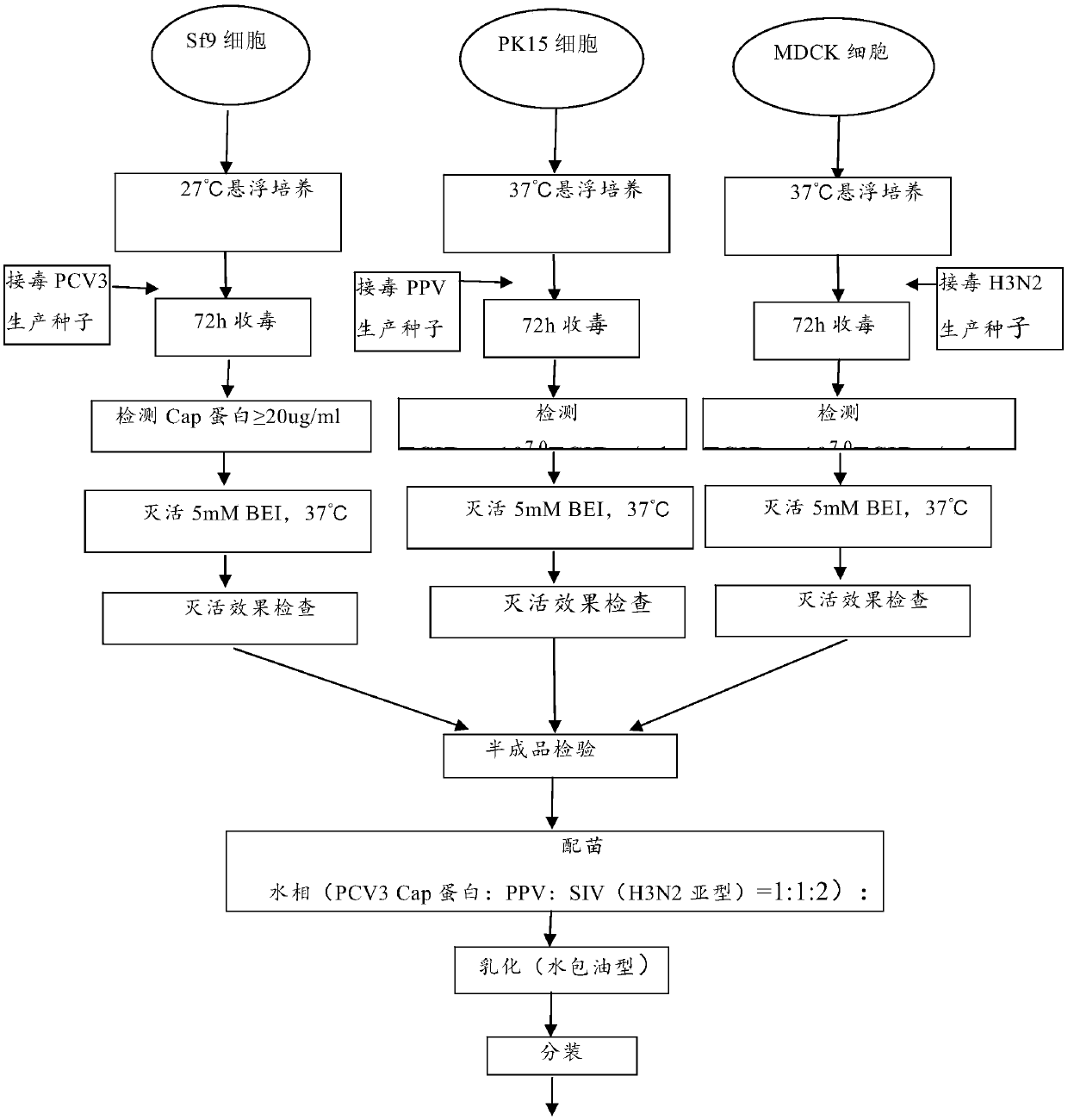
[0028] Embodiment 1 expresses the preparation of PCV3 type Cap protein recombinant baculovirus vector, PPV and S IV (H3N2 subtype) triple inactivated vaccine
[0029] 1 Obtaining Antigen Solution of Porcine Circovirus Type 3 Recombinant Baculovirus
[0030] 1.1 Construction of recombinant baculovirus vector expressing PCV3 type Cap protein
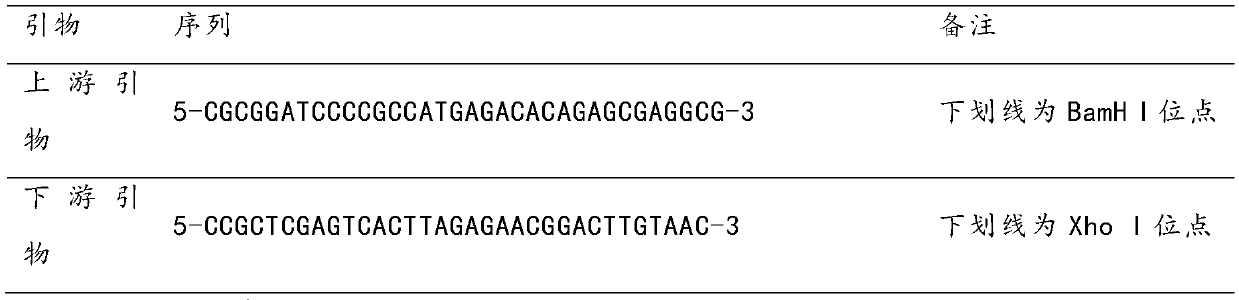
[0031] Use the primers in the following table 1 to amplify the Cap protein gene (ORF2) from the PCV3 strain DNA extracted from the suspected porcine circovirus disease material in Jilin, and after recovery, digest the recovery product with BamH I and Xho I respectively , and then ligated with the same digested plasmid pOET1, the obtained recombinant plasmid was identified by restriction enzyme digestion and PCR, and it was determined that the recombinant plasmid pOET1-ORF2-ChS-1 was obtained. Sf9 insect cells (Invitrogen) were co-transfected with insect virus DNA (flashBACPrime) from Oxford Expression Technologies, UK, and transfer plasmi...
Embodiment 2 3
[0074] The preparation of embodiment 2 triple inactivated vaccines
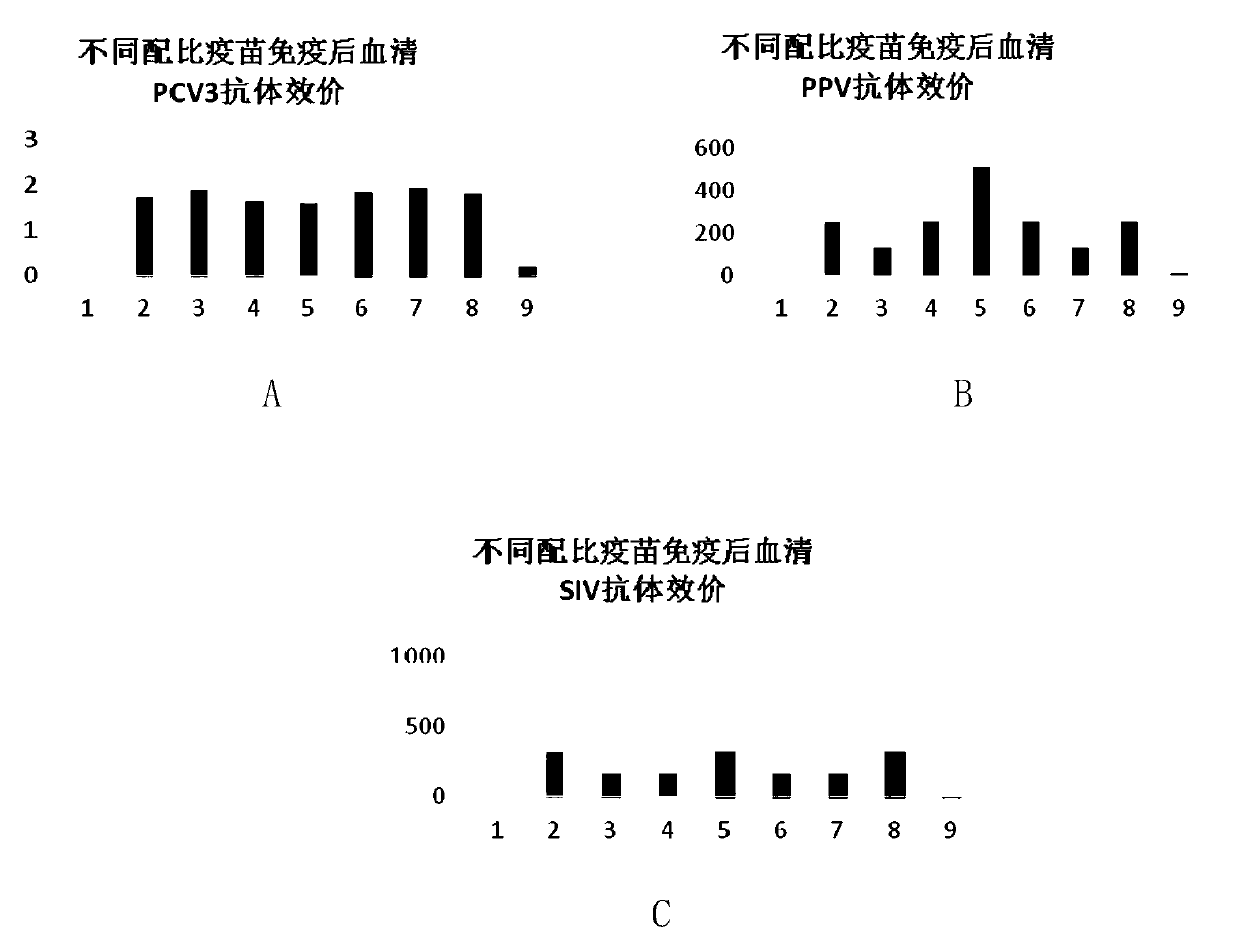
[0075] The preparation of porcine circovirus type 3 recombinant baculovirus antigen solution, porcine parvovirus L strain antigen solution and swine influenza H3N2 subtype HLJ strain antigen solution was prepared according to the method in Example 1, and the pig circovirus that passed the inactivation test was Virus type 3 recombinant baculovirus ChS-1 strain Cap protein antigen solution, PPV L strain antigen solution and SIV H3N2 subtype HLJ strain antigen solution were prepared in a ratio of 1:1:1 to prepare a triple inactivated vaccine, and the rest were mixed with Example 1 is the same.
Embodiment 3 3
[0076] The preparation of embodiment 3 triple inactivated vaccines
[0077] The preparation of porcine circovirus type 3 recombinant baculovirus antigen solution, porcine parvovirus L strain antigen solution and swine influenza H3N2 subtype HLJ strain antigen solution was prepared according to the method in Example 1, and the pig circovirus that passed the inactivation test was Virus type 3 recombinant baculovirus ChS-1 strain Cap protein antigen solution, PPV L strain antigen solution and SIV H3N2 subtype HLJ strain antigen solution were prepared in a ratio of 2:1:1 to prepare a triple inactivated vaccine, and the rest were mixed with Example 1 is the same.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com