Cysteine fluorescent probe and preparation method thereof
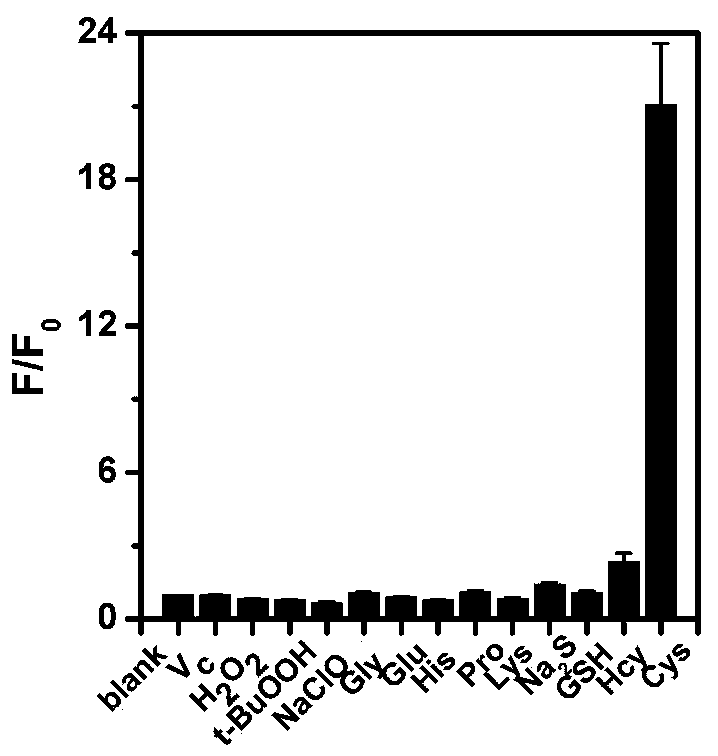
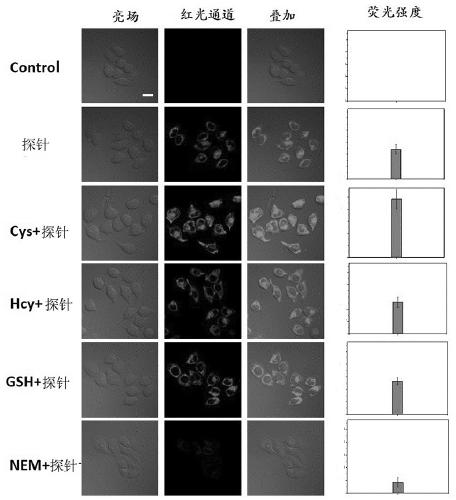
A fluorescent probe, cysteine technology, applied in the field of chemical analysis, can solve the problems of small Stokes shift, strong background signal interference, poor probe effect, etc., to achieve strong selectivity, high sensitivity, and improved imaging. quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
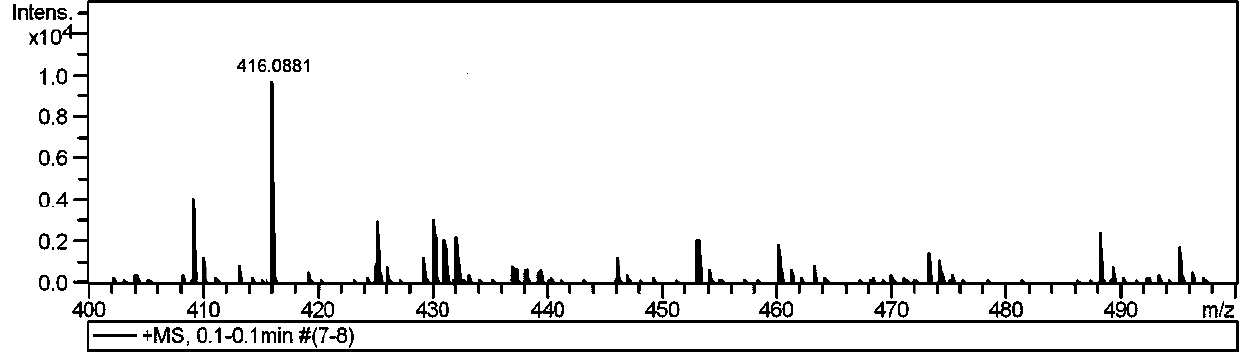
[0027] See figure 1 . Under argon protection, reactant 1 (0.23 g, 1.02 mmol) and reactant 2 (0.20 g, 1.05 mmol) were mixed in a Schlenk tube, and ammonium acetate (0.005 g, 0.05 mmol) was added as a catalyst, and then 2 mL tetrahydrofuran and 0.5 mL ethanol were used as reaction solvents, and stirred at 20 °C for 24 h. After the solvent was removed by rotary evaporation, it was purified by silica gel column chromatography, and the eluent was dichloromethane / acetone (20 / 1, v / v). A cysteine fluorescent probe was obtained, 0.29 g, with a yield of 75%. ESI-HRMS: [M+Na]+, [C25H15NO4Na]+, Calculated: 416.0899; Found: 416.0881.
Embodiment 2
[0029] Under argon protection, reactant 1 (0.15 g, 0.66 mmol) and reactant 2 (0.16 g, 0.853 mmol) were mixed in a Schlenk tube, and ammonium acetate (0.05 g, 0.65 mmol) was added as a catalyst, and then 2 mL Tetrahydrofuran and 0.5 mL methanol were used as reaction solvents, and stirred overnight at 60 °C. After the solvent was removed by rotary evaporation, it was purified by silica gel column chromatography, and the eluent was dichloromethane / acetone (20 / 1, v / v). 0.21 g of a cysteine fluorescent probe was obtained with a yield of 78%. ESI-HRMS: [M+Na]+, [C25H15NO4Na]+, Calculated: 416.0899; Found: 416.0883.
Embodiment 3
[0031] Under argon protection, reactant 1 (0.34 g, 1.52 mmol) and reactant 2 (0.41 g, 1.45 mmol) were mixed in a Schlenk tube, and ammonium acetate (0.12 g, 1.61 mmol) was added as a catalyst, and then 2.5 mL Ethanol was used as the reaction solvent and stirred at 80°C for 3h. After the solvent was removed by rotary evaporation, it was purified by silica gel column chromatography, and the eluent was dichloromethane / acetone (20 / 1, v / v). 0.45 g of a cysteine fluorescent probe was obtained with a yield of 78%. ESI-HRMS: [M+Na]+,[C25H15NO4Na]+, Calculated: 416.0899; Found: 416.0907.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com