Preparing method for range limiting type nanometer zero-valent iron-nickel composite
A nano-zero-valent iron and complex technology, applied in metal processing equipment, transportation and packaging, etc., to prevent surface passivation, prevent agglomeration, and promote the effective transfer of electrons to the surface of contaminants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A method for preparing a confined nanometer zero-valent iron-nickel composite, comprising the steps of:
[0024] (1) Ferric nitrate and nickel nitrate are dissolved in 20mL of organic solvent N,N-dimethylformamide in a molar ratio of 2:1 to obtain solution A, and the organic ligand ethylenediaminetetraacetic acid (with moles of iron salt ratio of 0.5:1) was dissolved in 20mL N,N-dimethylformamide to obtain solution B;
[0025] (2) Add solution A to solution B quickly, stir evenly, and centrifuge, wash the solid obtained after centrifugation with DMF, and then vacuum dry at 60°C to obtain the iron-nickel complex;
[0026] (3) Put the iron-nickel complex in a tube furnace, in a nitrogen atmosphere, control the heating rate to 2°C / min, the gas flow rate to 50mL / min, raise the temperature to 1000°C, keep the temperature for calcination for 3h, and then cool to room temperature , that is.
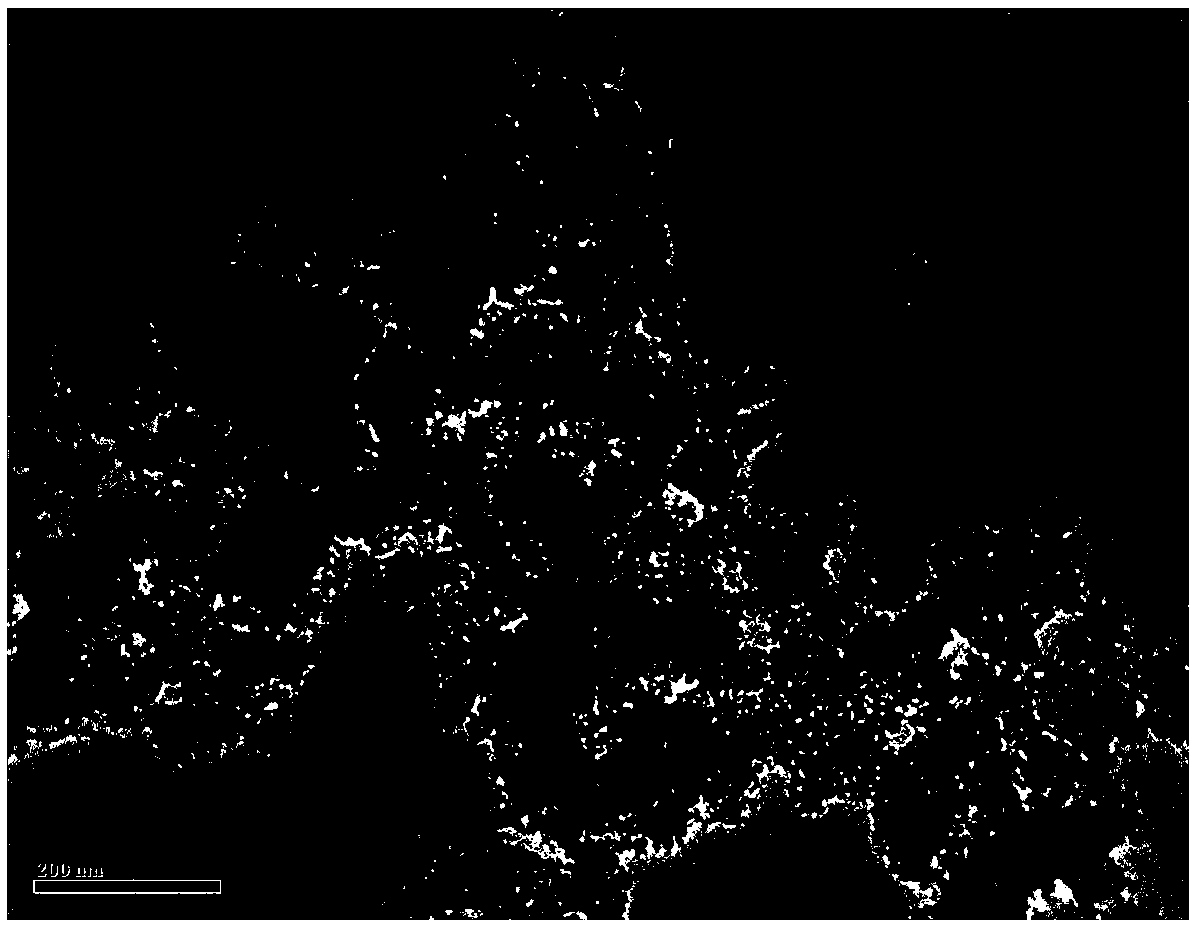
[0027] figure 1 The transmission electron microscope image of the confinement-type...
Embodiment 2
[0029] A method for preparing a confined nanometer zero-valent iron-nickel composite, comprising the steps of:
[0030] (1) Ferric chloride and nickel chloride are dissolved in 20mL of organic solvent N,N-dimethylformamide at a molar ratio of 5:1 to obtain solution A, and the organic ligand terephthalic acid (with iron salt) The molar ratio is 1:1) was dissolved in 20mLN,N-dimethylformamide to obtain solution B;
[0031] (2) Add solution A to solution B quickly, stir evenly, and centrifuge, wash the solid obtained after centrifugation with DMF, and then vacuum dry at 60°C to obtain the iron-nickel complex;
[0032] (3) Put the iron-nickel complex in a tube furnace, in a nitrogen atmosphere, control the heating rate to 5°C / min, the gas flow rate to 50mL / min, raise the temperature to 800°C, keep the temperature for calcination for 2h, and then cool to room temperature , that is.
Embodiment 3
[0034] A method for preparing a confined nanometer zero-valent iron-nickel composite, comprising the steps of:
[0035] (1) Ferric sulfate and nickel sulfate are dissolved in 20mL organic solvent N,N-dimethylformamide in molar ratio 10:1, obtain solution A, organic ligand triethylamine (the molar ratio with iron salt is 0.8:1) was dissolved in 20mL N,N-dimethylformamide to obtain solution B;
[0036] (2) Add solution A to solution B quickly, stir evenly, and centrifuge, wash the solid obtained after centrifugation with DMF, and then vacuum dry at 60°C to obtain the iron-nickel complex;
[0037] (3) Put the iron-nickel complex in a tube furnace, in a nitrogen atmosphere, control the heating rate to 1°C / min, the gas flow rate to 50mL / min, raise the temperature to 600°C, keep the temperature for calcination for 1h, and then cool to room temperature , that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com