High-rate stable lithium nickel phosphate positive electrode material and preparation method thereof
A lithium nickel phosphate and positive electrode material technology, applied in the field of lithium-ion batteries, can solve the problems of poor ion conductivity, poor high-rate stability, easy structure distortion and cracking, etc., and achieve low cost, simple process, excellent ion conductivity and The effect of conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
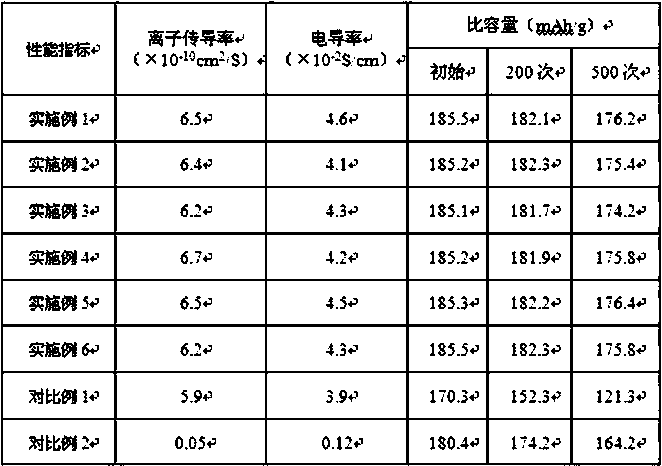
Embodiment 1
[0032]Put the ITO conductive glass into acetone, ethanol, and deionized water successively at room temperature, and ultrasonically treat them for 5 minutes respectively, and then put them into an electric constant temperature drying oven, and dry them at 50°C for 2 hours; The mixed solution of trisodium citrate hydrate, 1.50mL of n-propanol and 17.70mL of deionized water was left to stand for 50min to obtain the electrolyte; connected to the three-electrode system, the working electrode was made of ITO conductive glass, and the reference electrode was made of Ag|AgCl. Select a platinum sheet for the counter electrode, add the prepared electrolyte to the electrolytic cell, apply a voltage of -0.3V to the working electrode with an electrochemical workstation, and last for 300s, a layer of reddish-brown substance is formed on the ITO conductive glass, stop applying After voltage, remove the ITO conductive glass from the working electrode, wash off the electrolyte on the surface wi...
Embodiment 2
[0038] Put the ITO conductive glass into acetone, ethanol, and deionized water successively at room temperature, and ultrasonically treat them for 5 minutes respectively, and then put them into an electric constant temperature drying oven, and dry them at 50°C for 2 hours; The mixed solution of trisodium citrate hydrate, 1.50mL of n-propanol and 17.70mL of deionized water was left to stand for 40min to obtain the electrolyte; connected to the three-electrode system, the working electrode was made of ITO conductive glass, and the reference electrode was made of Ag|AgCl. Select a platinum sheet for the counter electrode, add the prepared electrolyte to the electrolytic cell, apply a voltage of -0.3V to the working electrode with an electrochemical workstation, and last for 300s, a layer of reddish-brown substance is formed on the ITO conductive glass, stop applying After voltage, remove the ITO conductive glass from the working electrode, wash off the electrolyte on the surface w...
Embodiment 3
[0041] Put the ITO conductive glass into acetone, ethanol, and deionized water successively at room temperature, and ultrasonically treat them for 5 minutes respectively, and then put them into an electric constant temperature drying oven, and dry them at 50°C for 2 hours; The mixed solution of trisodium citrate hydrate, 1.50mL of n-propanol and 17.70mL of deionized water was left to stand for 50min to obtain the electrolyte; connected to the three-electrode system, the working electrode was made of ITO conductive glass, and the reference electrode was made of Ag|AgCl. Select a platinum sheet for the counter electrode, add the prepared electrolyte to the electrolytic cell, apply a voltage of -0.3V to the working electrode with an electrochemical workstation, and last for 300s, a layer of reddish-brown substance is formed on the ITO conductive glass, stop applying After voltage, remove the ITO conductive glass from the working electrode, wash off the electrolyte on the surface w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com