Method of preparing high-activity uranium trioxide by thermal denitration of uranyl nitrate
A technology of uranyl nitrate and uranium trioxide, applied in the direction of uranium oxide/hydroxide, etc., can solve the problems of influence and poor activity of uranium oxide, and achieve the advantages of short process flow, good product activity and overcoming poor product activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
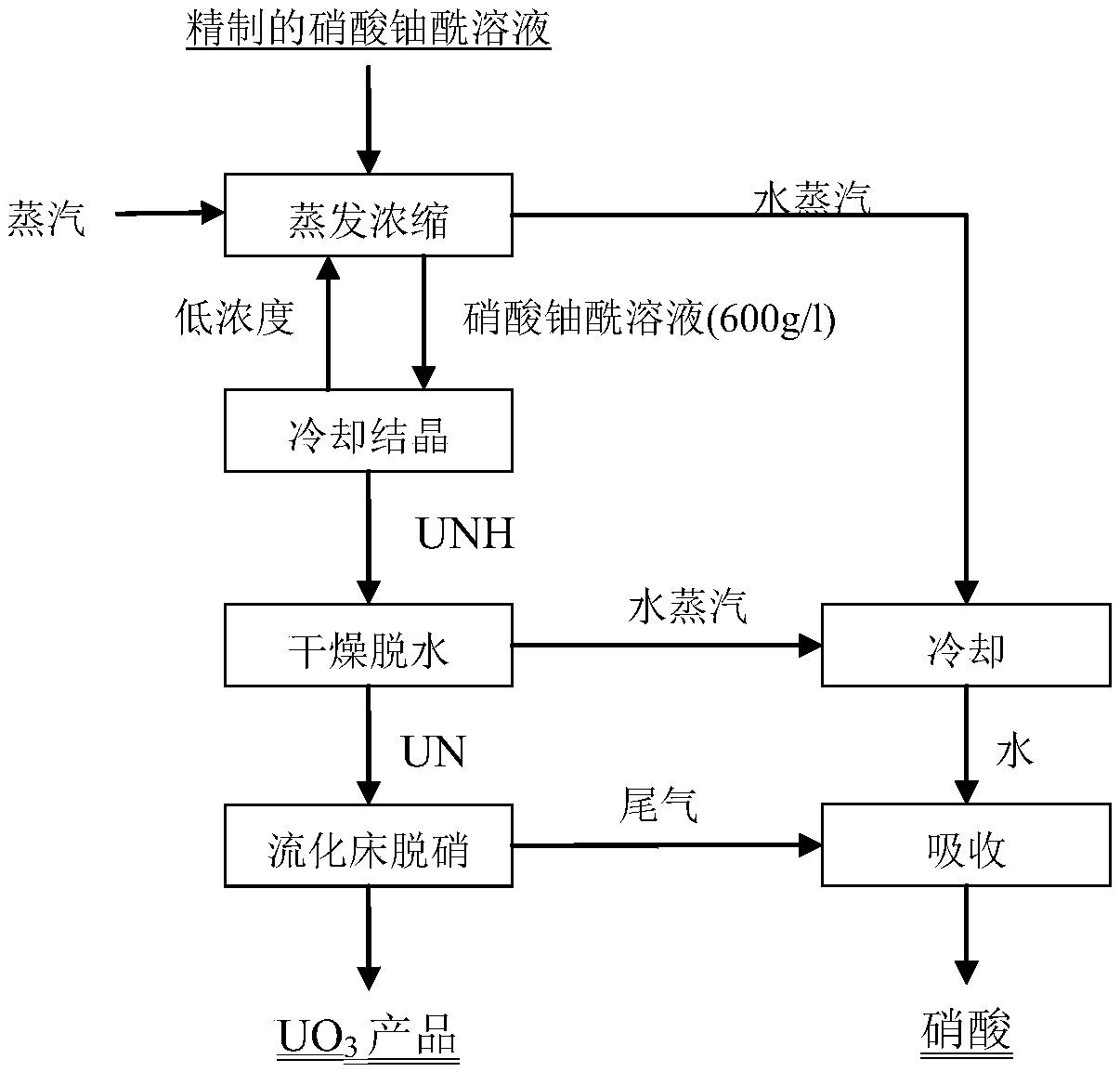
[0040] (1) The refined uranyl nitrate solution ([U]=85g / l, [HNO 3 ]=4mol / l, miscellaneous items meet the nuclear standard) with 2.0m 3 The / h flow rate is input into the MVR evaporator, and the temperature is controlled at 88°C to obtain a concentrated uranyl nitrate solution with a solubility of 580g / l;
[0041] (2) The concentrated uranyl nitrate solution is input into the cooling crystallizer, the stirring frequency is controlled to 50r / min, the cooling rate is 1°C / min, the final crystallization temperature is 40°C / min, and the crystallization is completed in 1.5h to generate UNH crystals;
[0042] (3) UNH crystals are input into a microwave dryer, the feed stirring frequency is 20Hz, and the dehydration temperature is controlled at 170°C to generate UN powder, which is stored in a vacuum sealed tank;
[0043] (4) The UN powder is conveyed with nitrogen, and the calcination temperature is controlled at 680 ° C in a microwave fixed bed to generate UO 3 Product, product qua...
Embodiment 2
[0045] (1) The refined uranyl nitrate solution ([U]=90g / l, [HNO 3 ]=3.8mol / l, miscellaneous items meet the nuclear standard) with 1.5m 3 The / h flow rate is input into the MVR evaporator, and the temperature is controlled at 90°C to obtain a concentrated uranyl nitrate solution with a solubility of 590g / l;
[0046] (2) The concentrated uranyl nitrate solution is input into the cooling crystallizer, the stirring frequency is controlled to 50r / min, the cooling rate is 1°C / min, the final crystallization temperature is 40°C / min, and the crystallization is completed in 1.5h to generate UNH crystals;
[0047] (3) UNH crystals are input into a microwave dryer, the feed stirring frequency is 20Hz, and the dehydration temperature is controlled at 175°C to generate UN powder, which is stored in a vacuum sealed tank;
[0048] (4) The UN powder is transported with nitrogen, and the calcination temperature is controlled at 690 ° C in a microwave fixed bed to generate UO 3 Product, produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com