N-butyl-4-hydroxyl-1,8-naphthalimide-3-formaldehyde-(2-pyridine)hydrazone and application
A naphthalimide and butyl technology, which is applied in the fields of organic synthesis and iron ion detection, can solve the problems of complex operation, low selectivity, low detection sensitivity, etc., and achieves simple operation method, good selectivity, and detection sensitivity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis and characterization of N-butyl-4-hydroxy-1,8-naphthalimide-3-formaldehyde-(2-pyridine)hydrazone (L):
[0034]Dissolve 0.045g (0.15mmol) of N-butyl-4-hydroxyl-1,8-naphthalimide-3-carbaldehyde and 0.02g (0.18mmol) of 2-hydrazine pyridine in 8mL of methanol and heat to 65 After reflux at ℃ for 1 h, solids precipitated out after cooling to room temperature, filtered with suction, and washed with a small amount of methanol to obtain a yellow solid L (0.054 g, yield 92.7%), L is N-butyl-4-hydroxyl-1,8- Naphthalimide-3-carbaldehyde-(2-pyridine)hydrazone. 1 H NMR (DMSO-d 6 )δ: 11.48(s,1H), 8.68(d,1H), 8.63(s,1H), 8.55(s,1H), 8.49(d,1H), 8.23(d,1H), 7.83(t,1H ),7.75(t,1H),6.99(d,1H),6.89(t,1H),4.05(t,2H),1.61(m,2H),1.35(m,2H),0.93(t,3H) .HRMS(ESI):calcd.for C 22 h 21 N 4 o 3 389.1614 (L+H), found: 389.1615.
Embodiment 2
[0036] Determination of Spectral Properties of N-Butyl-4-Hydroxy-1,8-Naphthalimide-3-Formaldehyde-(2-Pyridine) Hydrazone (L)
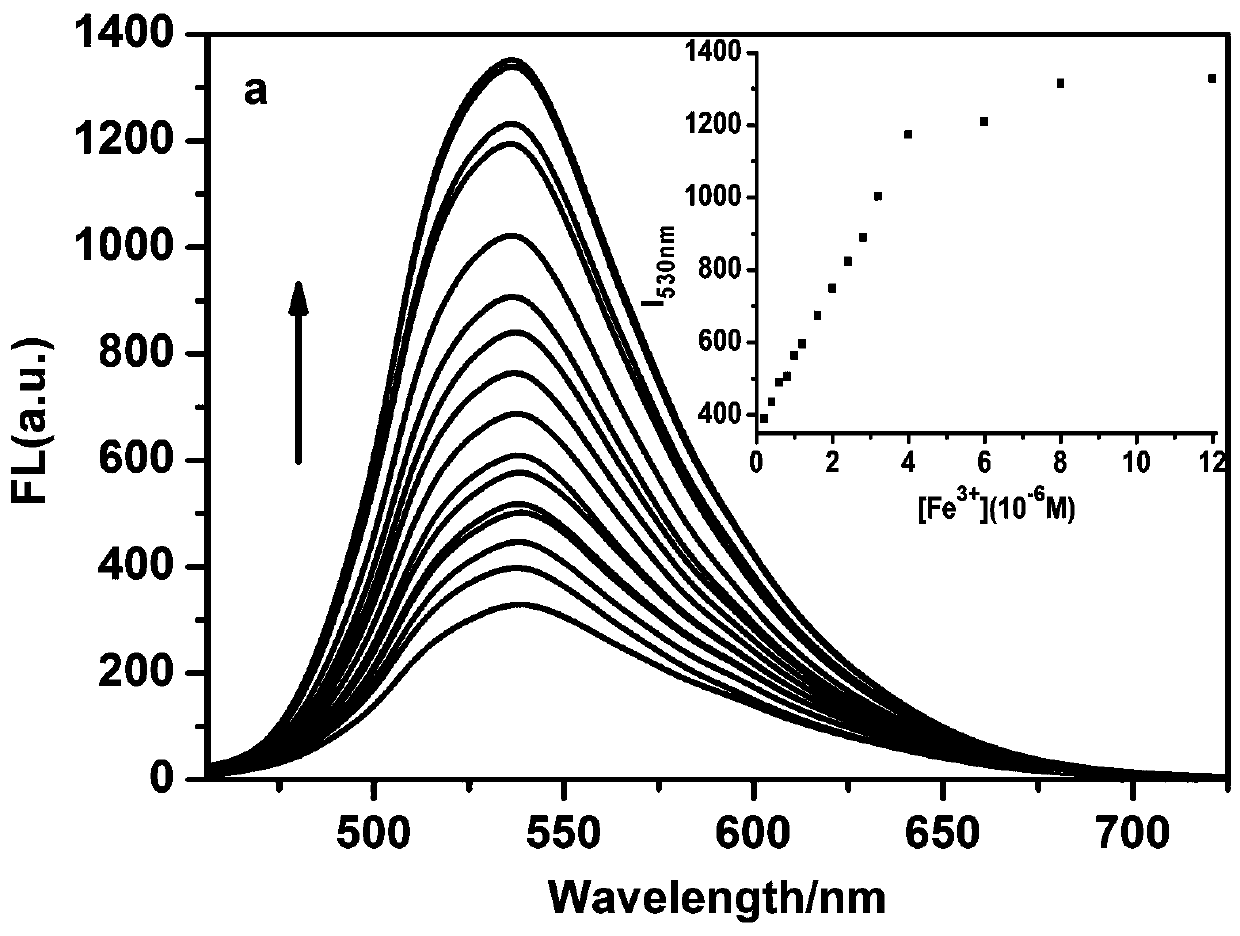
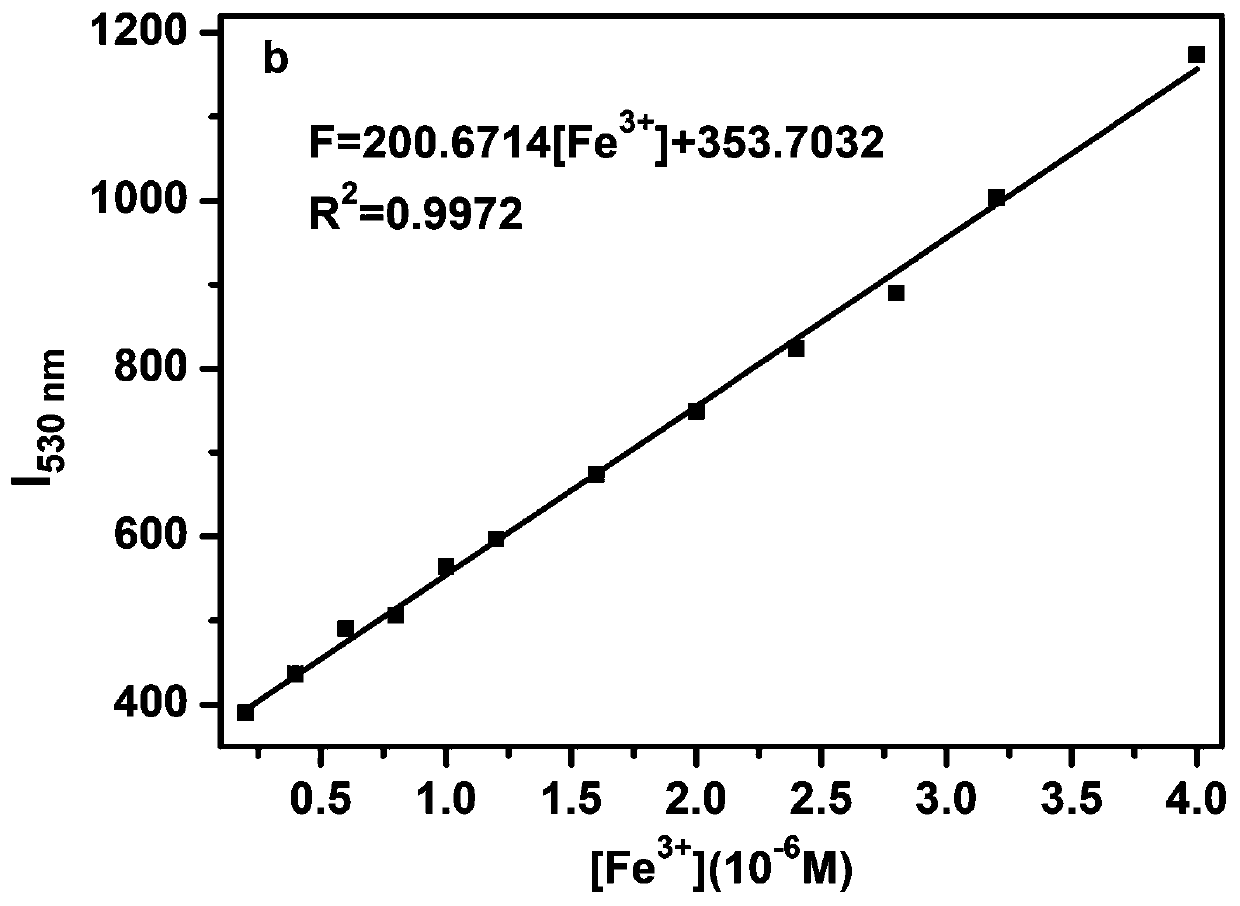
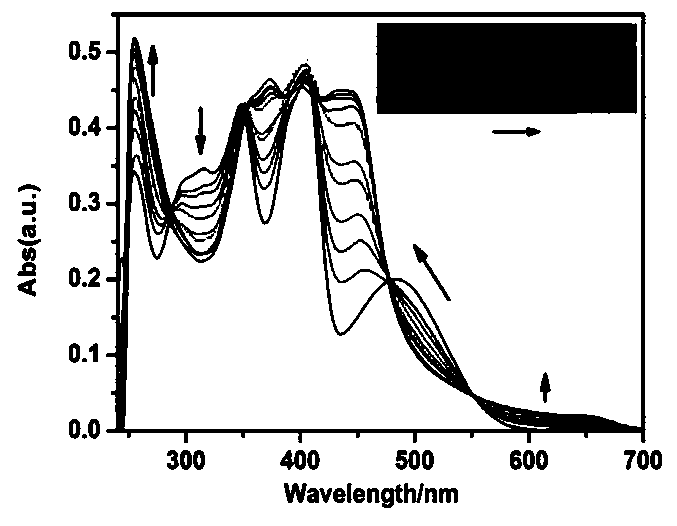
[0037] The L prepared in Example 1 was dissolved in methanol to obtain 1 × 10 -3 M's stock solution, dispensing 10 -2 The metal ion stock solution of M was used for the metal ion selectivity determination. The stock solution was further diluted to an appropriate concentration for fluorescence detection. The pH of the detection system was adjusted with 0.05mol / L Tris-hydrochloric acid buffer solution, and the pH of the buffer solution was adjusted to 7.4 with hydrochloric acid. The detection system is DMSO:H 2 O=7:3 (v / v, pH=7.4). The fluorescence titration experiment process is as follows: take 25 μL of the L stock solution in a colorimetric tube, add a corresponding volume of 1×10 -3 Fe of M 3+ For the stock solution, add 1 mL of buffer and secondary water to make the total volume of water 1500 μL, then dilute to 5 mL with DMSO, shake well and u...
Embodiment 3
[0043] The N-butyl-4-hydroxyl-1,8-naphthalimide-3-formaldehyde-(2-pyridine) hydrazone (L) prepared in embodiment 1 is to Fe 3+ Determination of selectivity experiments
[0044] In DMSO:H containing 5 μM of probe L prepared in Example 1 2 O=7:3 (v / v, pH=7.4) solution is added with an equal amount of K + , Ca 2+ , Na + ,Mg 2+ ,Al 3+ ,Zn 2+ , Ag + ,Hg 2+ ,Pb 2+ ,Cu 2+ , Fe 3+ , Fe 2+ ,Cd 2+ , Ni 2+ ,Cr 3+ ,Co 2+ ions, Fe only 3+ The fluorescence of the system is significantly enhanced, indicating that the probe L has an effect on Fe 3+ are very selective, such as Figure 4 shown. Interference experiments of coexisting ions showed that common metal ions on Fe 3+ The detection interference of L is very small, indicating that L detects Fe 3+ Has strong anti-interference ability, such as Figure 5 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com