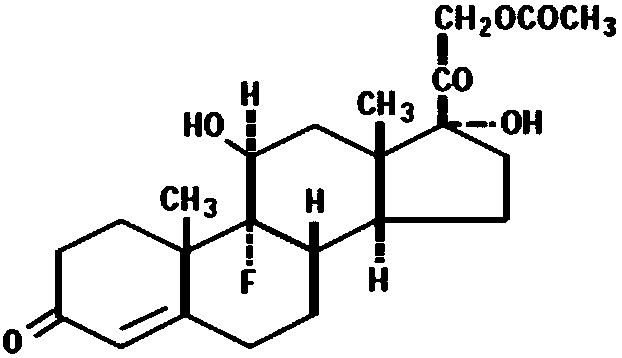
Fludrocortisone acetate oral cavity instant film agent and preparation method thereof
A technology of fludrocortisone acetate and oral quick-dissolving film, applied in the field of medicine, can solve problems such as difficulty in research and development, difficulty in swallowing ordinary tablets, and dangers, and achieves the advantages of accelerating drug absorption, accelerating drug dissolution rate, and reducing impurities. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
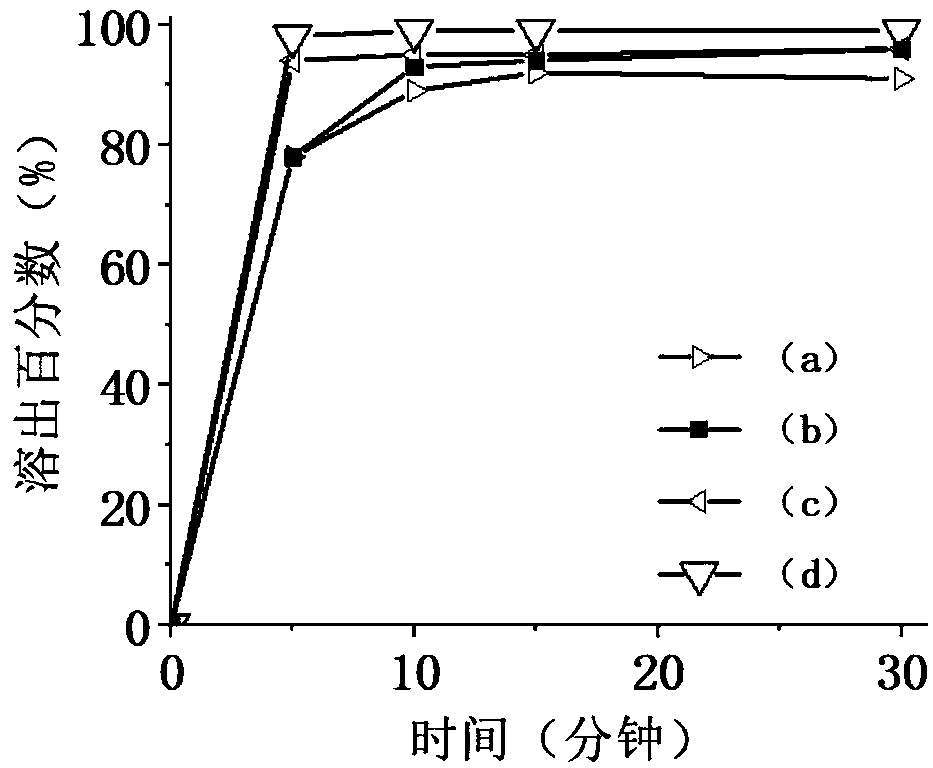
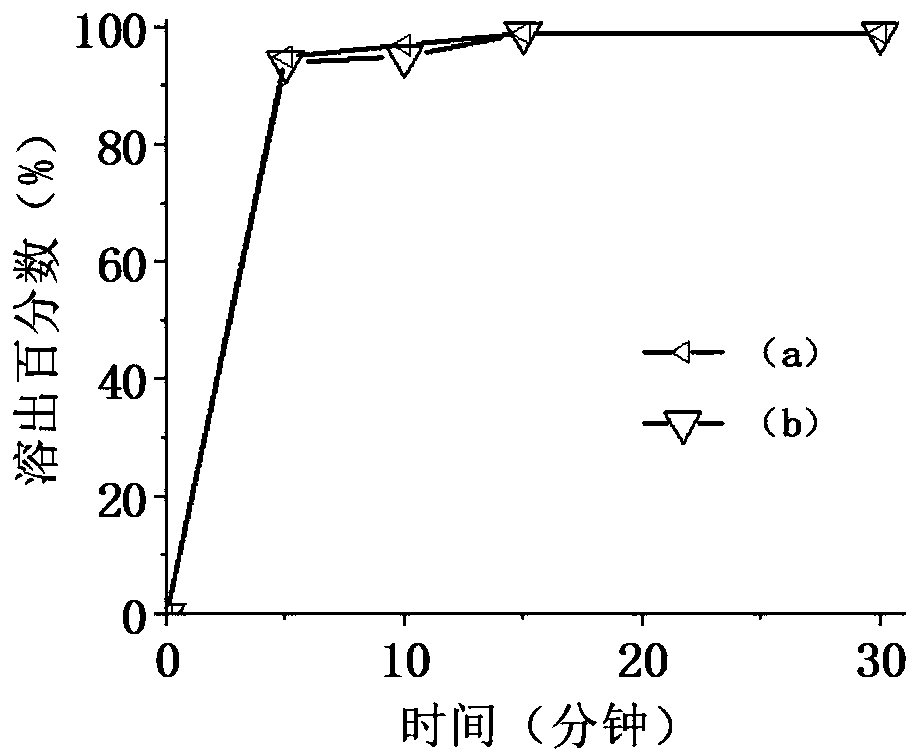
Image
Examples
Embodiment 1
[0042] The prescription quantity is 1000 tablets (specification 0.1mg: 10mg).
[0043] Table 1:
[0044] fludrocortisone acetate
0.1g
Hydroxypropyl-β-cyclodextrin
1.5g
polyoxyethylene
0.1g
Hypromellose (E50LV)
4.9g
2.4g
1.0g
90ml
Absolute ethanol*
10ml
[0045] *Used in prescription but removed during processing.
[0046] 1. Preparation of inclusion compound
[0047] Accurately weigh 1.5g of hydroxypropyl-β-cyclodextrin, add 10ml of purified water and stir to dissolve; take fludrocortisone acetate and dissolve it in 10ml of ethanol to obtain fludrocortisone acetate solution; Add the ethanol solution of fludrocortisone acetate dropwise to the aqueous solution of hydroxypropyl-β-cyclodextrin; after dropping, sonicate for 5 minutes to obtain the inclusion complex of fludrocortisone acetate and hydroxypropyl-β-cyclodextrin substance solution.
[0048] 2. Prepar...
Embodiment 2
[0055] The prescription quantity is 1000 tablets (specification 0.1mg: 10mg).
[0056] Table 2:
[0057] fludrocortisone acetate
0.1g
polyoxyethylene
0.1g
Hypromellose (E50LV)
4.9g
3.9g
1.0g
90ml
Absolute ethanol*
10ml
[0058] *Used in prescription but removed during processing.
[0059] (1) Dissolve fludrocortisone acetate in 10 ml of absolute ethanol to obtain fludrocortisone acetate solution.
[0060] (2) After dissolving mannitol and dextran in 20ml of purified water, stir to dissolve.
[0061] (3) Then the solutions obtained in steps (1) and (2) were mixed and stirred for 3-5 minutes.
[0062] (4) Next, add polyoxyethylene solution dissolved in 20 ml of purified water to the solution obtained in step (3), and stir evenly.
[0063] (5) Dissolve hypromellose in 50ml of water, mix the obtained hypromellose solution with the solution obtained in step (4), and sti...
Embodiment 3
[0067] The prescription quantity is 1000 tablets (specification 0.1mg: 10mg).
[0068] table 3:
[0069] fludrocortisone acetate
0.1g
polyoxyethylene
0.1g
Hypromellose (E50LV)
5.8g
4.0g
purified water*
90ml
Absolute ethanol*
10ml
[0070] *Used in prescription but removed during processing.
[0071] (1) Dissolve fludrocortisone acetate in 10 ml of absolute ethanol to obtain fludrocortisone acetate solution.
[0072] (2) Dissolve dextran in 20ml of purified water, and stir to dissolve.
[0073] (3) Then the solutions obtained in steps (1) and (2) were mixed and stirred for 3-5 minutes.
[0074] (4) Next, add polyoxyethylene solution dissolved in 20 ml of purified water to the solution obtained in step (3), and stir evenly.
[0075] (5) Disperse the hypromellose in 50ml of hot water, after cooling, continue to stir until the hypromellose is completely dissolved. Mix the hypromellose solution obtained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com