Hollow tubular carbon nitride photocatalyst and its preparation method and application
A catalyst, tubular technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc. Additives are difficult to completely remove and other problems, to achieve a large number of rapid preparation, industrial application, separation and migration rate fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
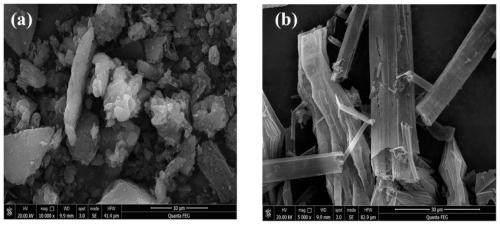
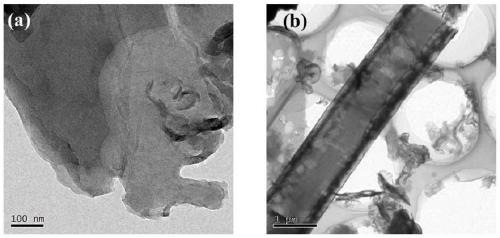
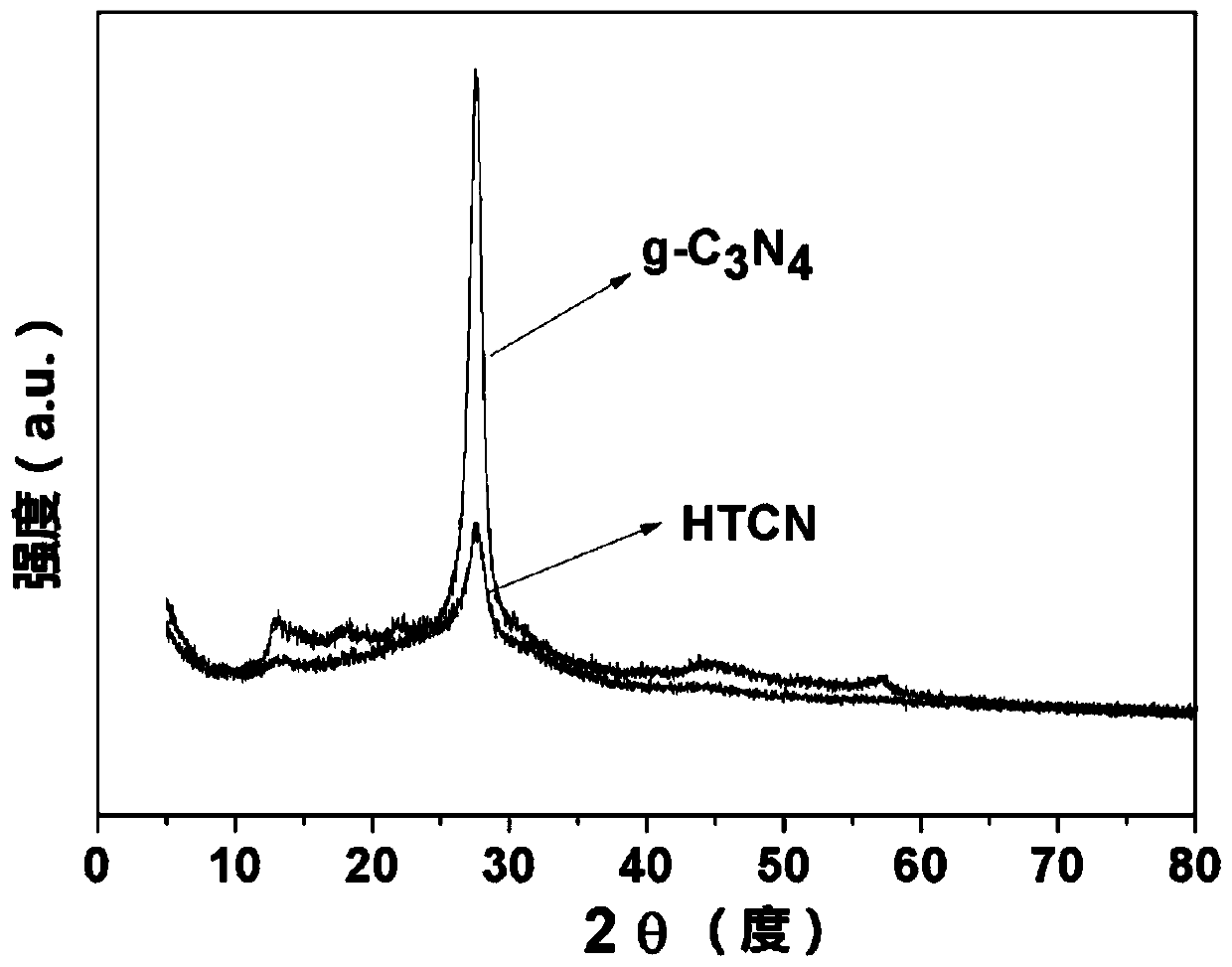
[0034] A hollow tubular carbon nitride photocatalyst is prepared by using urea and melamine as raw materials through hydrothermal and calcination, wherein the molar ratio of urea and melamine is 3:1, and its preparation method specifically includes the following steps:
[0035] S1. Take 9.56g of urea and 6g of melamine for grinding, dissolve in 70ml of deionized water, ultrasonicate for 1h, stir for 8h, and mix well to obtain a mixed solution.
[0036] S2. Transfer the mixed solution obtained in step S1 to a 100mL autoclave, and carry out a hydrothermal reaction at 180°C for 24 hours. After natural cooling, the resulting reaction product is washed 3 times with water and ethanol, and filtered (specifically, the filter), and dried at 70°C for 12 hours to obtain a precursor.
[0037] S3. Put the precursor obtained in step S2 into a crucible, place it in a muffle furnace, heat it to 550°C at a heating rate of 2.3°C / min for calcination, and keep it at 550°C for 240min. After natura...
Embodiment 2
[0050] Application of a hollow tubular carbon nitride photocatalyst in degrading organic pollutants, specifically utilizing the hollow tubular carbon nitride photocatalyst prepared in Example 1 and the monomer carbon nitride prepared in Comparative Example 1 to degrade water Tetracycline in, including the following steps:
[0051] Get the hollow tubular carbon nitride photocatalyst obtained in Example 1 and the monomeric carbon nitride obtained in Comparative Example 1, each 30mg, join 50mL respectively, in the tetracycline solution that concentration is 20mg / L, in darkroom After stirring for 60 minutes, the solution was placed under the condition of visible light (300W xenon lamp) to carry out photocatalytic reaction for 2 hours to complete the degradation of tetracycline in water.
[0052] During the photocatalytic reaction, take 3ml tetracycline solution every 15 minutes in the first hour, and take 3ml tetracycline solution every 30 minutes in the next hour, measure the con...
Embodiment 3
[0055] Application of a hollow tubular carbon nitride photocatalyst in degrading organic pollutants, specifically utilizing the hollow tubular carbon nitride photocatalyst prepared in Example 1 and the monomer carbon nitride prepared in Comparative Example 1 to degrade water Rhodamine B in, comprises the following steps:
[0056] Get the hollow tubular carbon nitride photocatalyst that makes in the embodiment 1 and the monomer carbon nitride that make in the comparative example 1, each 20mg, join respectively in the rhodamine B solution that 30mL, concentration are 10mg / L, in Stir in the dark room for 60 minutes, and then place the solution under the condition of visible light (300W xenon lamp) to carry out photocatalytic reaction for 1 hour, and complete the degradation of Rhodamine B in water.
[0057] During the photocatalytic reaction, 3 mL of rhodamine B solution was taken every 15 minutes, and the content of rhodamine B in the solution was measured with a UV-visible spec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com