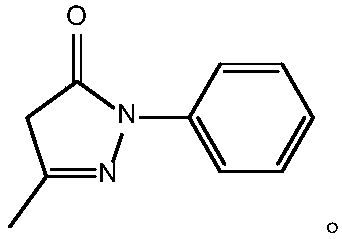
Preparing method of edaravone
A technology for edaravone and phenylhydrazine, which is applied in the field of preparation of heterocyclic compounds, can solve the problems of high content, difficult removal of phenylhydrazine and its derivatives, and the like, achieves low impurity content, and reduces phenylhydrazine residues and derivatives thereof. The content of impurities, the effect of short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The preparation method of edaravone provided by the present invention includes the following steps:
[0021] 1) Reaction of phenylhydrazine and ethyl acetoacetate with a molar ratio of 1:1.019 to 1.080 in an organic solvent at 25 to 30°C for 1 to 4 hours, and then removing the solvent to obtain an oil;
[0022] 2) Mix the oil obtained in step 1) uniformly with acetic acid, perform reflux reaction at 75-77°C for 6-10 hours, then remove the unreacted acetic acid, add alcohol solvent to mix uniformly, and separate solid-liquid to obtain.
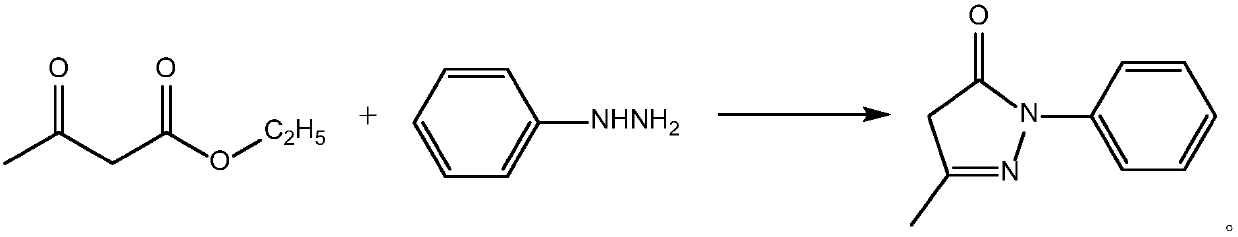
[0023] The chemical reaction involved in the preparation method of edaravone of the present invention is:
[0024]
[0025] Through process research, it is found that when there is too much ethyl acetoacetate, more impurities are produced. When the feeding ratio of phenylhydrazine to ethyl acetoacetate is 1:1.019~1.080, there are fewer impurities and it also meets the requirements of economic production.
[0026] Preferably, an inert gas is used t...
Embodiment 1
[0056] The preparation method of edaravone of this embodiment includes the following steps:
[0057] 1) Add anhydrous ethanol to a 500L reactor, add 70kg of phenylhydrazine (about 647mol) while continuously stirring the anhydrous ethanol, pass nitrogen protection, and stir to make it completely dissolved. Cool the circulating water, add 88.5kg ethyl acetoacetate (approximately 680mol) dropwise, keep the temperature not higher than 40℃, after the dropwise addition, keep the temperature at 25℃ and react for 4h, monitor the reaction by TLC; concentrate and distill the ethanol under reduced pressure to obtain oily Thing
[0058] 2) Add 147 kg of acetic acid to the oily substance obtained by concentrating, stir, pass nitrogen protection, heat up to 105° C. to reflux, reflux for 10 hours, monitor the reaction by TLC, and distill off the acetic acid under reduced pressure to obtain an oily substance.
[0059] 120kg of ethanol was added to the obtained oily substance and stirred at room tem...
Embodiment 2
[0066] The preparation method of edaravone of this embodiment includes the following steps:
[0067] 1) Add anhydrous ethanol to a 500L reactor, add 70kg of phenylhydrazine (about 647mol) while continuously stirring the anhydrous ethanol, pass nitrogen protection, and stir to make it completely dissolved. Cool the circulating water, add 85.9kg ethyl acetoacetate (approximately 660mol) dropwise, keep the temperature not higher than 40°C, after the dropwise addition, keep the temperature at 25°C and react for 4h, monitor the reaction by TLC; concentrate under reduced pressure and distill the ethanol to obtain an oily Thing
[0068] 2) Add 147 kg of acetic acid to the oily substance obtained by concentrating, stir, pass nitrogen protection, heat up to 110° C. to reflux, reflux for 6 hours, monitor the reaction by TLC, and evaporate the acetic acid under reduced pressure to obtain an oily substance.
[0069] Add 160 kg of ethanol to the obtained oil, and stir at room temperature. After ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com