Reactive dye substituted by benzene-containing sulfonamide and derivative of benzene-containing sulfonamide, and preparation method of reactive dye
A technology of reactive dyes and benzenesulfonamide, applied in the direction of reactive dyes, azo dyes, organic dyes, etc., to achieve the effect of broad application prospects and high light fastness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
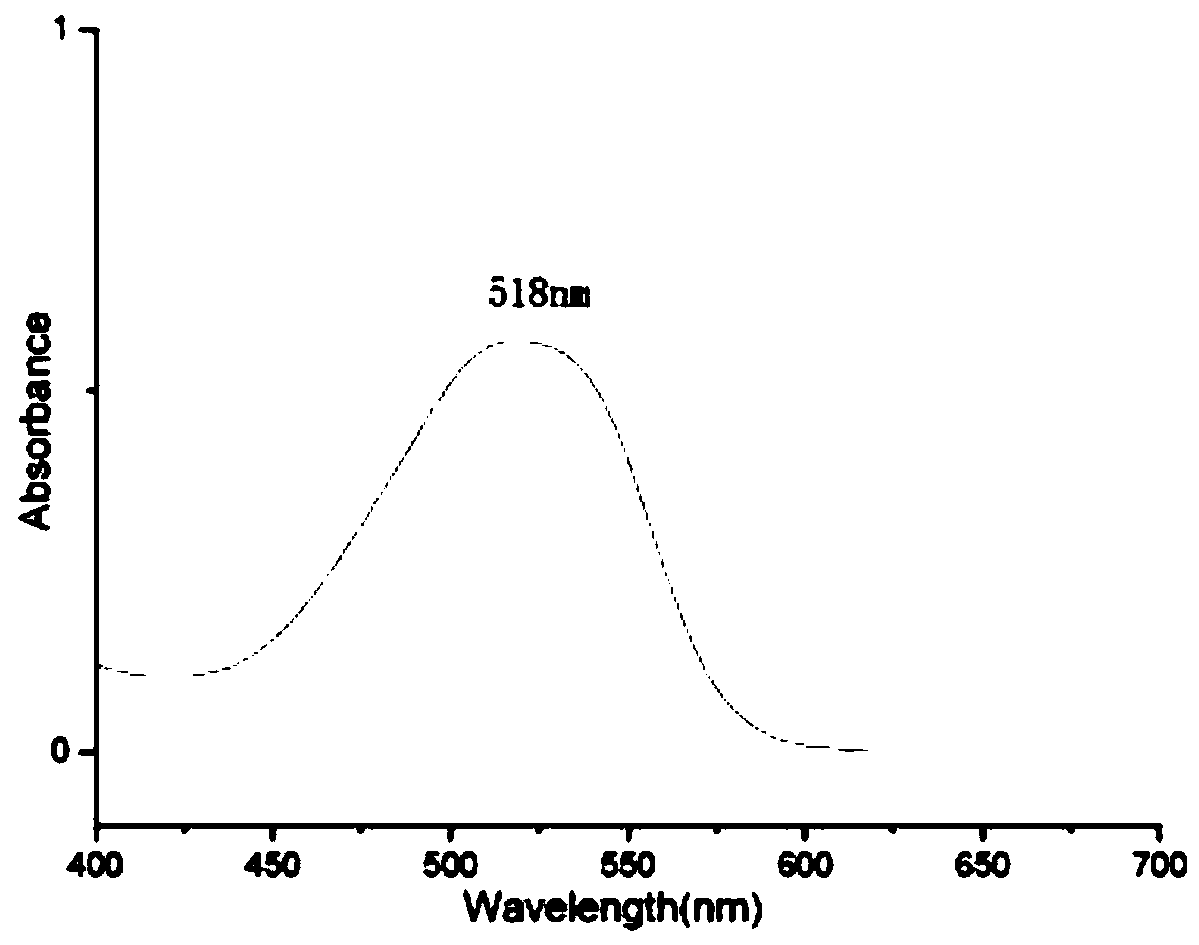
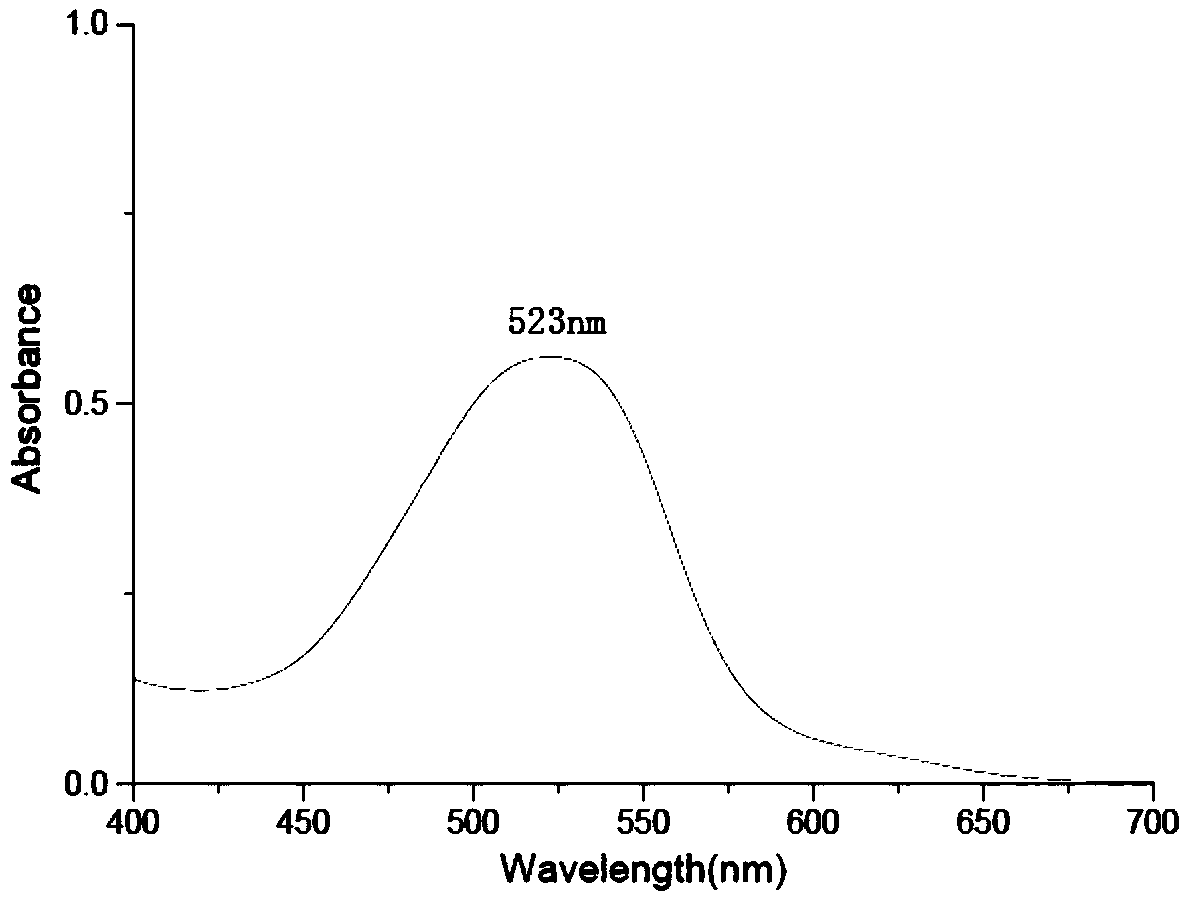
Image
Examples
Embodiment 1
[0085] Cyanuric chloride condensation reaction: Weigh 3.14g of benzenesulfonamide, add 40mL of water and 0.8g of sodium hydroxide, heat up to 20°C and stir until dissolved to obtain a mixed solution; add dropwise to the mixed solution at 0-5°C 1.84g of cyanuric chloride dissolved in 20mL of acetone was added dropwise within 40min. React at 0-5°C until the shrinkage reaction is completed. After the shrinkage is completed, add 10% hydrochloric acid to adjust the pH of the reaction solution to <3 to precipitate unreacted benzenesulfonamide, and filter to obtain a clear filtrate. Add 4.0 g of H acid to the above reaction filtrate during the second shrinkage, control the reaction temperature at 25° C., pH 6-7, and control the reaction to the end point by thin-layer chromatography to obtain the condensation product.
[0086] Diazotization reaction: weigh 0.01 mol of p-ester solid, adjust pH 5-6 and dissolve in 20 mL of water. 0.73 g of sodium nitrite was added to dissolve it. Add ...
Embodiment 2~3
[0090] Substitute p-carboxybenzenesulfonamide and p-toluenesulfonamide for benzenesulfonamide in Example 1 respectively, and use the same synthesis method to obtain the structural dyes shown in Examples 2 and 3.
Embodiment 4
[0092] The cyanuric chloride condensation reaction is identical with embodiment 1 reaction steps, replaces 4.0g H acid among the embodiment 1 with 2.15g J acid during two shrink steps.
[0093] Diazotization reaction: same as the preparation method of Example 1.
[0094] Coupling reaction: gradually add the above-mentioned diazo solution to the above-mentioned dicondensation product solution dropwise, adjust the coupling pH to 7-8 with a 10% mass fraction of sodium carbonate solution, control the coupling temperature at 0-5°C, and detect it by the percolation ring method at intervals of 10 minutes The reactions of the diazo component and the coupling component were combined with TLC to detect the progress of the reaction. After the coupling reaction is finished, adjust the reaction pH to about 7, and add buffer salt (Na 2 HPO 3 -NaH 2 PO 3 , 1%, w / v), and then slowly add about 5% of the reaction system quality potassium acetate solid salting out in batches until a water ri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com