Kit for detecting lipoprotein phospholipase A2 protein concentration
A phospholipase and lipoprotein technology, which is applied in the field of kits for detection of lipoprotein phospholipase A2 (Lp-PLA2) protein concentration, can solve the problem of not truly reflecting the true concentration level of Lp-PLA2, the detection limitations of limited sample types, etc. To achieve the effect of facilitating large-scale promotion and application, satisfying emergency and outpatient rapid diagnosis, and expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of a kit for detecting the protein concentration of lipoprotein phospholipase A2
[0033] 1. Preparation of calibrators and quality controls
[0034] The concentration of Lp-PLA2 antigen was determined by traceability, and diluted with calibrator diluent to prepare Lp-PLA2 calibrator and Lp-PLA2 quality control substance. For example, the concentration of the calibrator is 0, 50, 100, 500, 800, 1000ng / ml, and the concentration of the quality control substance is 400ng / ml.
[0035] The specific components of the calibrator diluent used are: 20mM HEPES (4-hydroxyethylpiperazineethanesulfonic acid), 300mM NaCl, 1% bovine serum, 0.5mM Proclin-300, and its pH is 6.0.
[0036] 2. Preparation of pretreatment solution
[0037] The pretreatment solution uses 25mM Tris as the matrix solution, which contains 1.9mM pyridine, 0.5-5.5% X-100; also includes 150mM NaCl, 1% sucrose, 5% glycerol, 0.1% BSA, 0.05% Tween-20 and 0.2% Proclin-300, its pH is 7.0.
[0...
Embodiment 2
[0077] Example 2 Determination of Lp-PLA2 antigen in whole blood using the kit prepared in Example 1
[0078] The sample to be tested is whole blood from fingertips, and the specific steps are:
[0079] 1. Add 10 μl of Lp-PLA2 calibrator, Lp-PLA2 quality control substance and test sample to three reaction tubes respectively;
[0080] 2. Add 40 μl of pretreatment solution to each reaction tube and mix well;
[0081] 3. Add 50 μl enzyme conjugate working solution and 50 μl magnetic bead working solution to each test tube and mix well; react at 42°C for 3 minutes;
[0082] 4. Magnetically separate the solution after the reaction in step 3 to collect the magnetic beads; add 300 μl of cleaning solution to each reaction tube to wash the magnetic beads, repeat 3-5 times, and remove the cleaning solution;
[0083] 5. Add 100 μl of substrate solution to each reaction tube, mix well and detect the luminescence value.
[0084] 6. Use the concentration and luminescence value of the sta...
Embodiment 3
[0087] Embodiment 3 The performance measurement of kit of the present invention
[0088] 1. Linear verification
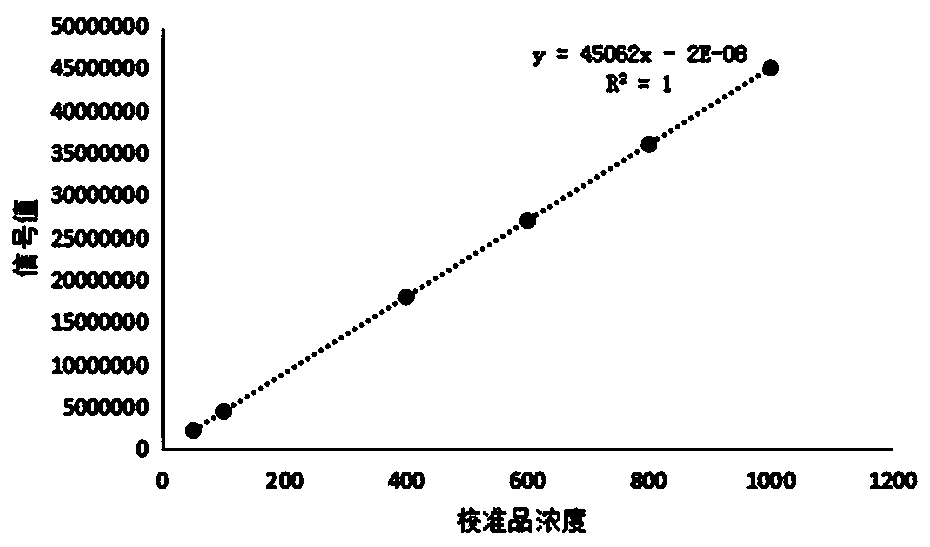
[0089] Take a traceable standard or quality control product with a concentration as high as possible, dilute the sample with normal saline, measure each point 3 times, take the average value, draw a regression line between the result and the expected concentration, and calculate the regression coefficient r > 0.99, It shows that the dilution linearity of the kit provided by the invention is good.
[0090] 2. Precision verification
[0091] Take a traceable serum high-value quality control product and a low-value quality control product, perform 10 tests on each quality control product, and calculate the average value and standard value of a total of 10 test results.
[0092]
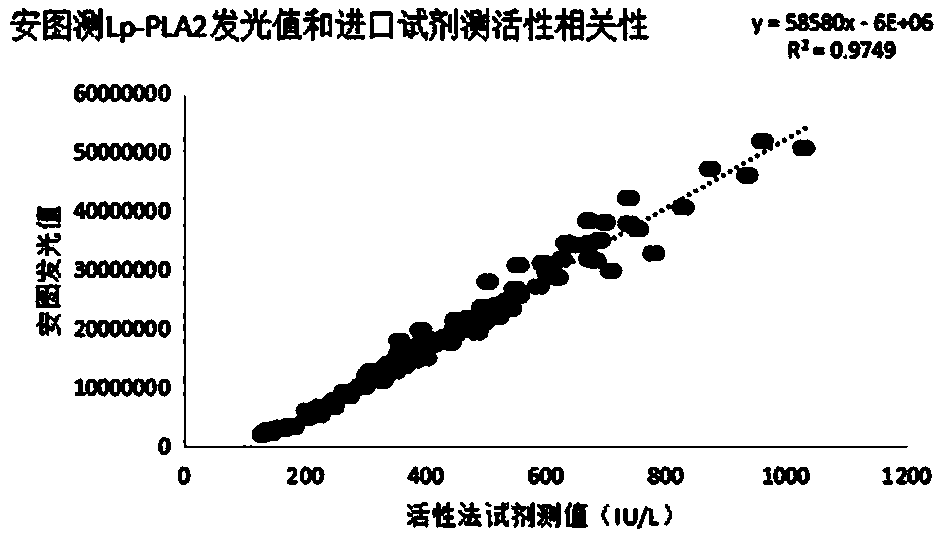
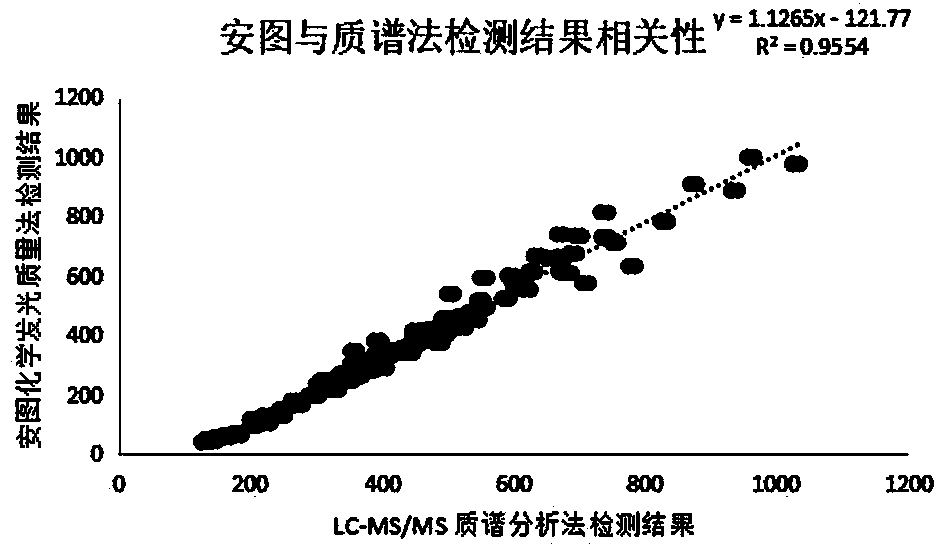
[0093] According to the coefficient of variation CV=(standard deviation / mean value)×100%, it is calculated: CV1 (350 ng / mL)=4%, CV2 (800 ng / mL)=3%. It can be known that the kit provided...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com