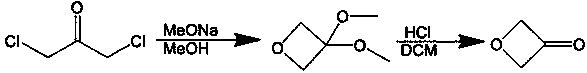
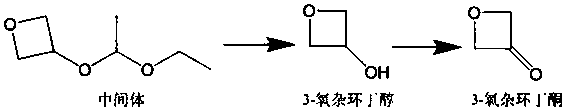
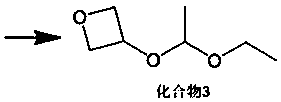
3-oxetanone synthesis method
A technology for oxetanone and a synthesis method, which is applied in the production of bulk chemicals, organic chemistry, etc., can solve problems such as unstable yield, achieve the effects of being environmentally friendly, saving purification steps, and avoiding the use of dangerous chemicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035](1) Add 140g of intermediate (purity 95%) and 280mL of methanol into a 500mL reaction flask, and slowly add the methanol solution of p-toluenesulfonic acid dropwise at 0°C (the methanol solution of p-toluenesulfonic acid is 0.46 g p-toluenesulfonic acid was dissolved in 5mL methanol), heated to 20°C, stirred for 1 hour, added sodium bicarbonate to neutralize to a pH value of 7.0-8.0, continued to stir for 1 hour, and then distilled off the solvent methanol to obtain The crude product of 3-oxetanol was 75.3g; the mass content of organic solvent I methanol in the crude product of 3-oxetanol was less than 1%; the mass content of 3-oxetanol was 81.5%.
[0036] (2) Add 425g of dichloromethane, 140g of NCS, 101g of sodium bicarbonate, 14g of tetrabutylammonium bromide and 7g of TEMPO into a 1L dry three-necked flask, and then add 75.3g of crude 3-oxetanol dropwise. After the dropwise addition was completed, stirring was continued at 30-40° C. for 2 hours. After the reaction m...
Embodiment 2
[0038] (1) Add 140g of the intermediate (purity 95%) and 280mL of ether into a 500mL reaction flask, and slowly add the ether solution of p-toluenesulfonic acid dropwise at 0°C (the ether solution of p-toluenesulfonic acid is 0.46 g p-toluenesulfonic acid was dissolved in 5mL ether), heated to 20°C, stirred and reacted for 1 hour, added potassium bicarbonate to neutralize to pH 7.0-8.0, continued to stir for 1 hour, and then distilled off the solvent ether to obtain 78.5 g of crude 3-oxetanol. The mass content of the organic solvent I methanol in the crude product of 3-oxetanol was less than 1%; the mass content of 3-oxetanol was 77.3%.
[0039] (2) Add 430g of dichloromethane, 350g of NBS, 121g of potassium bicarbonate, 7g of sodium bromide and 7g of TEMPO into a 1L dry three-necked flask, and then add 78.5g of crude 3-oxetanol dropwise. After the dropwise addition was completed, stirring was continued at 30-40° C. for 2 hours. After filtering the reaction mixture, it was d...
preparation example 1
[0045] (1) Add 139g of epichlorohydrin and 90g of glacial acetic acid into a 500mL reaction bottle, then add 0.6g of ferric chloride, raise the temperature to 70°C, and stir for 7 hours.
[0046] (2) Add 1.0g of p-toluenesulfonic acid to the reaction solution, slowly add 80g of vinyl ethyl ether dropwise, and control the dropping temperature at 40°C. After the dropwise addition, continue to stir and react at 40°C for 2 hours, then cool down to 0 0.9 g of p-toluenesulfonic acid was added, and the reaction was stirred at 40° C. for 4 hours to obtain a reaction solution.
[0047] (3) Add 345g of 50% NaOH solution into another 1000mL reaction bottle, raise the temperature to 110°C, slowly add the reaction liquid obtained in step (2) dropwise, and control the temperature during the dropping process at 110°C. Stir the reaction at 110°C for 7 hours, lower the temperature to 20°C, add 145g of water, 383g of dichloromethane, and 190g of saturated saline into the reaction bottle, stir a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com