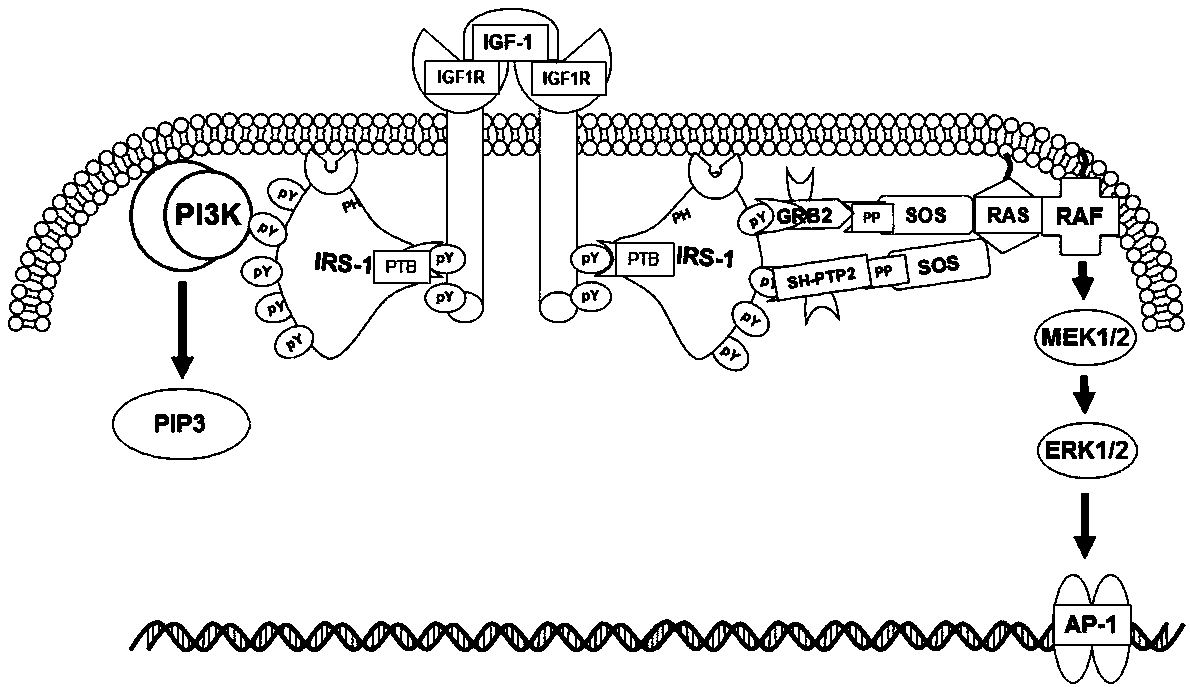
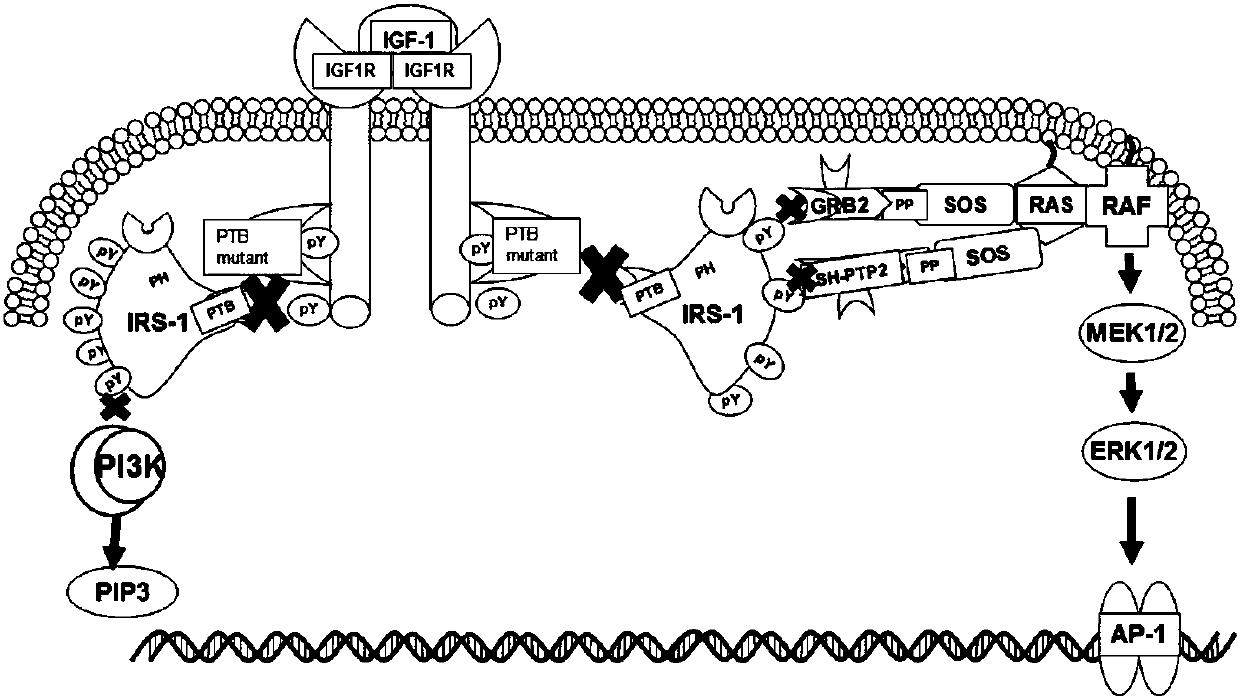
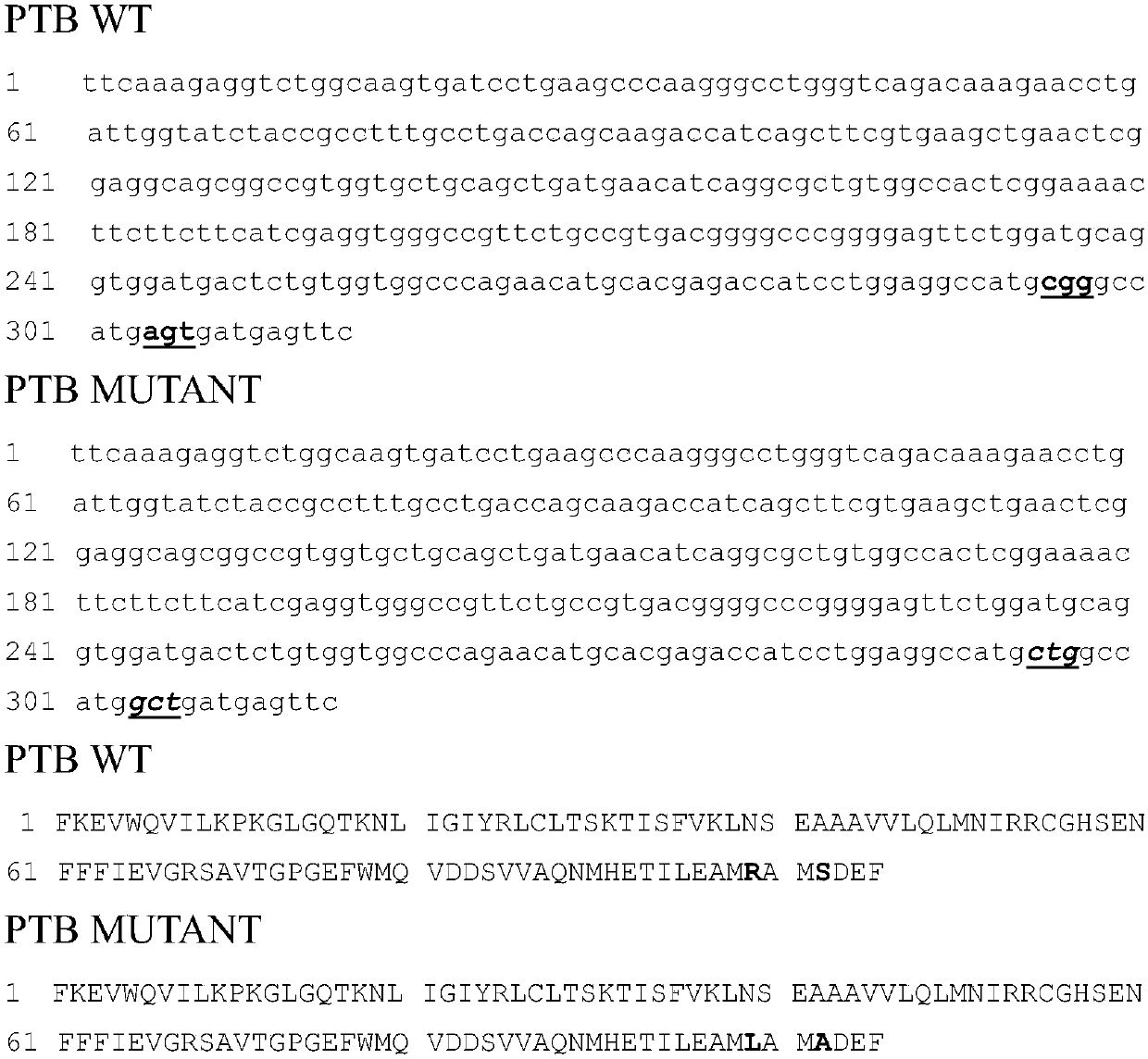
Mutant IRS-1 PTB domain proteins, and coding sequence and application thereof
A domain protein, IRS-1 technology, applied in the application field of PTB-MUTANT fusion gene and its prokaryotic expression vector, can solve the problems of high price, large molecular weight, drug resistance, etc., to improve affinity and reduce binding free energy. , the effect of enhancing affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. Construction of pGEX-4T2-PTB fusion gene expression vector
[0048] 1.1 Cloning of PTB and PTB-MUTANT domain genes
[0049] According to the PTB nucleotide sequence of IRS-1 in Genbank, such as image 3 As shown, the amplification primers are designed, and considering that the vector construction is a molecular cloning method using homologous recombination, appropriate homology arms should be added when designing the primers, which are F1 and R1 respectively. The specific primer sequences are as follows:
[0050] Forward primer F1: GATCTGGTTCCGCGTttcaaagaggtctggcaag34;
[0051] Reverse primer R1: GTCAGTCAGTCACGAgaactcatcactcatggcc34;
[0052] Using the cDNA of MCF7 cells as a template, using F and R as amplification primers respectively, the PTB domain gene was amplified by PCR. The PCR system was 2x PCR Mix 10ul, F1 1ul, R1 1ul, H 2 O 6ul, cDNA 2ul. The PCR program was 98°C for 2min, 98°C for 30s, 55°C for 30s, 72°C for 30s, and 35cycle.
[0053] The primer sequ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com