Kit for quantificationally and qualitatively detecting smooth muscle antibodies
A qualitative detection and smooth muscle technology, applied in biological testing, measuring devices, material inspection products, etc., to achieve high sensitivity, strong specificity, and high use value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of a kit for detecting anti-smooth muscle antibodies
[0050] 1. Preparation of magnetic particle reagents
[0051] 1. Prepare magnetic particle reagent storage buffer:
[0052] Add 1000ml of purified water, 6.05g of Tris and 9.0g of sodium chloride into the container and stir to dissolve; add 10g of bovine serum albumin, 1ml of Proclin300, 0.2g of Bro and 0.4g of MgCl·6H 2 Add O into the container and stir until completely dissolved; use 6M hydrochloric acid to adjust the pH to 7.4±0.1, prepare the storage buffer, and store it at 2-8°C for later use;
[0053] 2. Prepare magnetic particle reagent buffer 1:
[0054] Add 1000ml of purified water, 0.58g of sodium dihydrogen phosphate, 5.78g of disodium hydrogen phosphate, and 8.78g of sodium chloride into the container, stir and dissolve fully, measure the pH value between 7.0-7.5, and store at 2-8°C for later use;
[0055] 3. Prepare magnetic particle reagent buffer 2:
[0056] Add 1000ml of puri...
Embodiment 2
[0075] Embodiment 2 The detection method of kit of the present invention
[0076] 1. Sample requirements
[0077] 1. Use correct medical technology to collect serum or plasma samples: Serum samples can be collected using ordinary tubes or procoagulant tubes, and plasma samples can be collected using EDTA, heparin sodium, or sodium citrate anticoagulant tubes.
[0078] 2. The sediment and suspended matter in the sample may affect the test results, and should be removed by centrifugation, and the sample can only be used after confirming that it has not deteriorated.
[0079] 3. Samples with lipemia, hemolysis or turbidity cannot be used for the determination of this project.
[0080] 4. After the sample is collected, it should not be stored at room temperature for more than 8 hours; if it is not tested within 8 hours, the sample should be placed in a refrigerator at 2~8°C; if it needs to be stored or transported for more than 48 hours, it should be frozen at -20°C , avoid repe...
Embodiment 3
[0089] Embodiment 3 The performance test of the kit prepared by the present invention
[0090] 1. Blank limit,
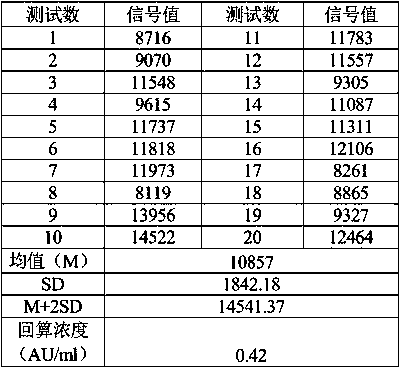
[0091] Use the zero-concentration calibrator as the sample for detection, repeat the measurement 20 times, obtain the luminescence value (RLU) of the 20 measurement results, calculate the mean (M) and standard deviation (SD), and obtain the RLU corresponding to M+2SD. The concentration value and signal value of each point of the calibrator are fitted according to the four-parameter lin-log method, and the RLU corresponding to M+2SD is substituted into the fitting curve to obtain the corresponding concentration value, which is the blank limit. The blank limit of this method requires no more than 5AU / ml. See Table 1 for blank limit data:
[0092] Table 1 Blank limit evaluation
[0093]
[0094] It can be seen from the data in Table 1 that the obtained blank limit is smaller than the required blank limit, which meets the needs of clinical use.
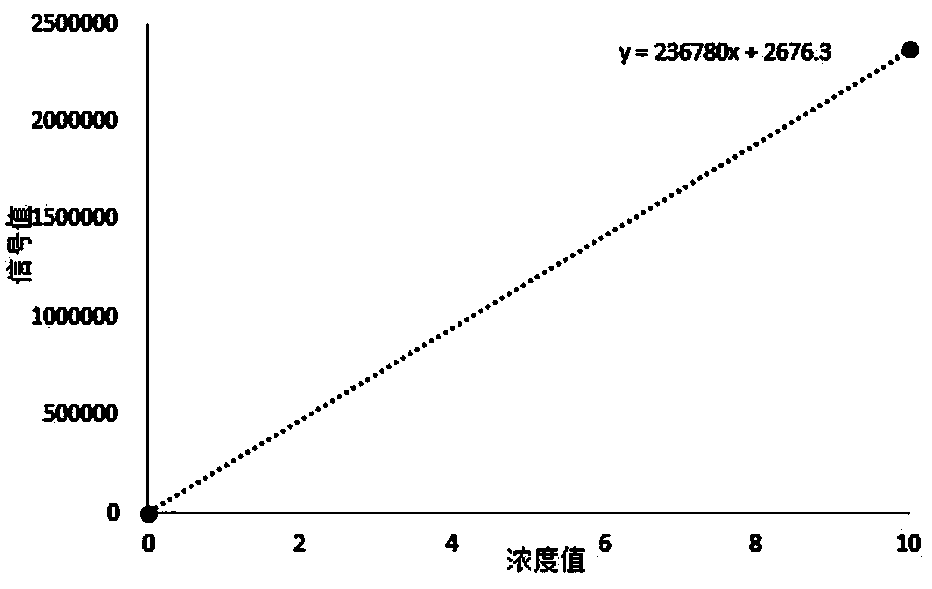
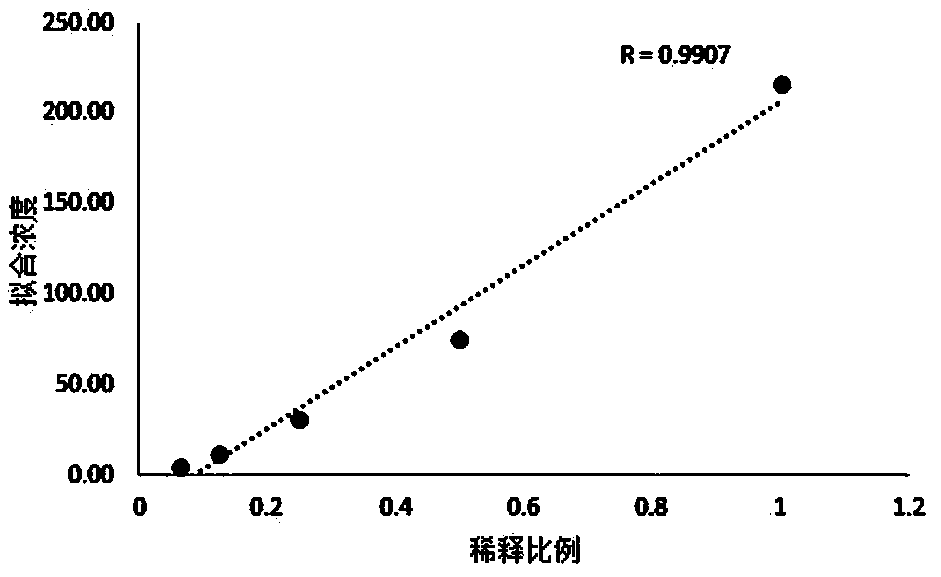
[0095] 2. Linear...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com