Medical hydrogel with radiation prevention effect
A hydrogel and medical technology, applied in the field of biomedicine, can solve the problems of wide molecular weight distribution, low long-term stability and unfavorable discharge of hyperbranched polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
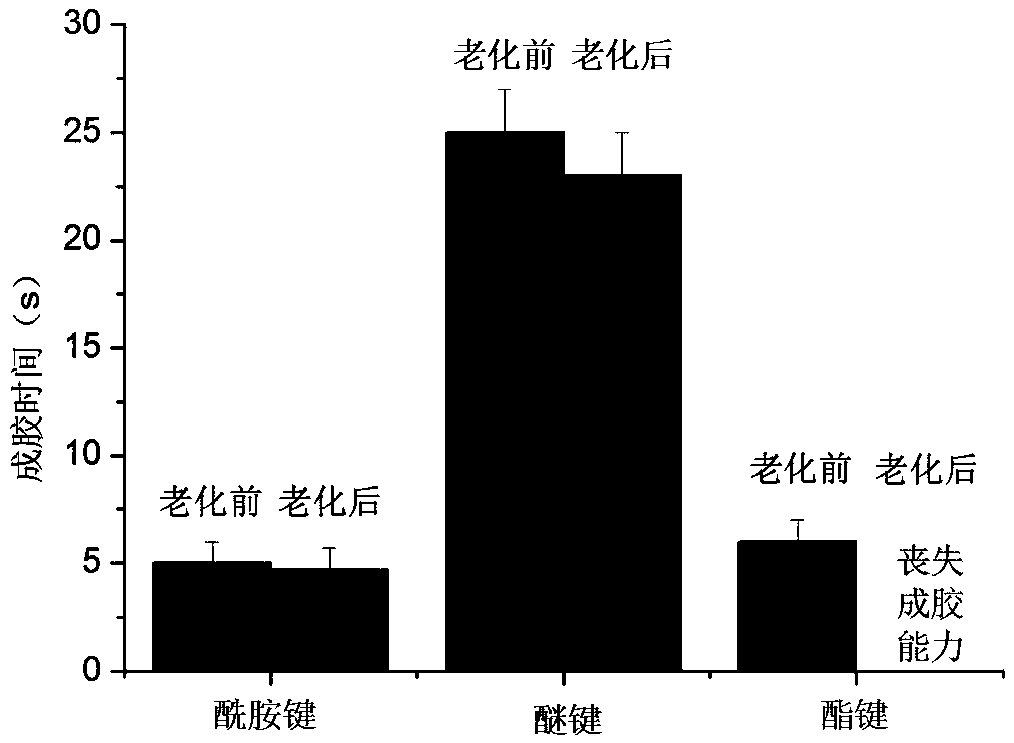
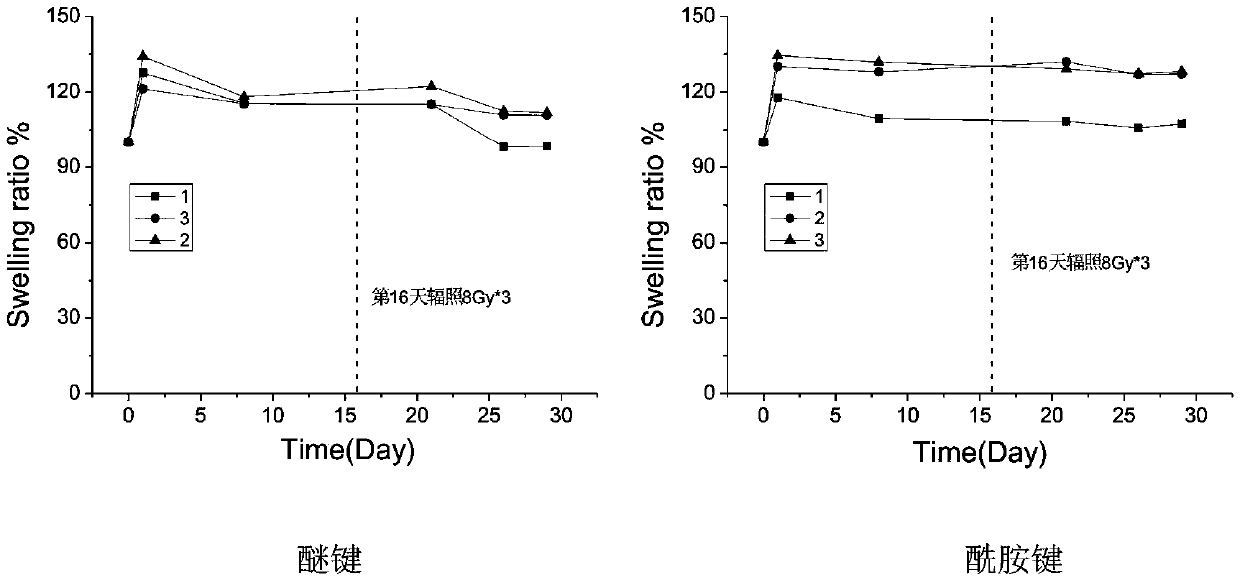
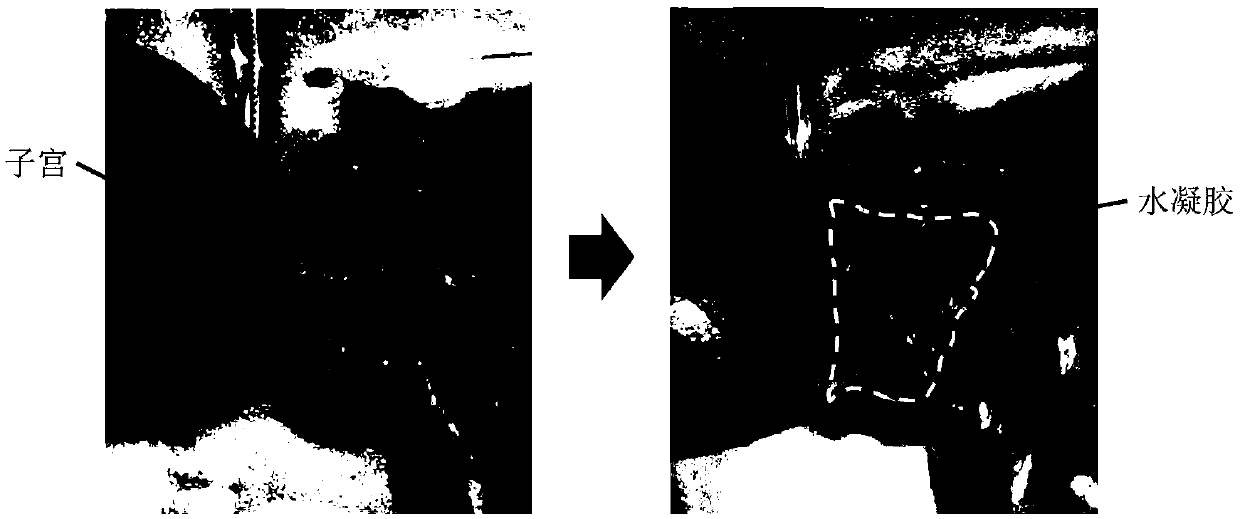
[0041] Example 1 The long-term gelation stability of ether bond, amide bond and ester bond-linked benzaldehyde group-terminated polyethylene glycol in aqueous solution 600mg of ester bond-connected benzaldehyde group-terminated 8-arm polyethylene glycol 8-PEG-amide-BA, amide bond connected to benzaldehyde group-terminated 8-arm polyethylene glycol 8-PEG-amide-BA and ether bond connected to benzaldehyde group-terminated 8-arm polyethylene glycol 8-PEG-O- BA was dissolved in 2 mL of 0.1M phosphate buffer solution (pH 7.4) as A solution; the configuration contained polylysine 2.75% (w / v) (the ratio of amino group to aldehyde group was 1:1) and polyethyleneimine (MW1.8K) 0.67% (w / v) (amino group to aldehyde group ratio is 0.4:1) phosphate buffer solution, as the B solution; mix the A and B solutions in equal volumes to obtain a viscous hydrogel , Record the initial gel time. Place the three A liquids in a constant temperature incubator at 60 degrees Celsius. After two months (equi...
Embodiment 2
[0042] Example 2 Gelling time and stability of hydrogels of different formulations in aqueous solutions
[0043] Dissolve 600 mg of amide bond-linked benzaldehyde group-terminated 8-arm polyethylene glycol 8-PEG-amide-BA (MW13.5K) in 2 mL of phosphate buffer (pH 7.4) as solution A; configuration is different The content of polylysine and polyethyleneimine (MW1.8K) in a phosphate buffer solution is used as the B solution; the A and B solutions are mixed in equal volumes to obtain a viscous hydrogel. In the following table, the amount ratio of polyethyleneimine and polylysine is represented by the molar ratio of the aldehyde groups in the star-shaped multi-arm polyethylene glycol terminated by their respective amino groups and aldehyde groups.
[0044]
[0045]
[0046] The results show that the content of polylysine significantly affects the stability of the hydrogel in aqueous solution. When the molar ratio of the amino group of polylysine to the aldehyde group of 8-PEG-amide-BA is...
Embodiment 3
[0048] Dissolve 400 mg of amide bond-linked benzene aldehyde group-terminated 8-arm polyethylene glycol 8-PEG-amide-BA (MW10K) in 2 mL of phosphate buffer (pH 7.4) as solution A; configure polylysine Acidic 2.44% (w / v) borate buffer solution (amino group to aldehyde group ratio 1:1), as B solution; mix A and B solutions in equal volumes to obtain a viscous hydrogel, gel time It is 25 seconds, and the in vitro stability exceeds 1 month.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com