Method for producing cyclohexylamine from aniline
A technology of cyclohexylamine and aniline, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of low yield and selectivity of cyclohexylamine, and achieve the effect of improving yield and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
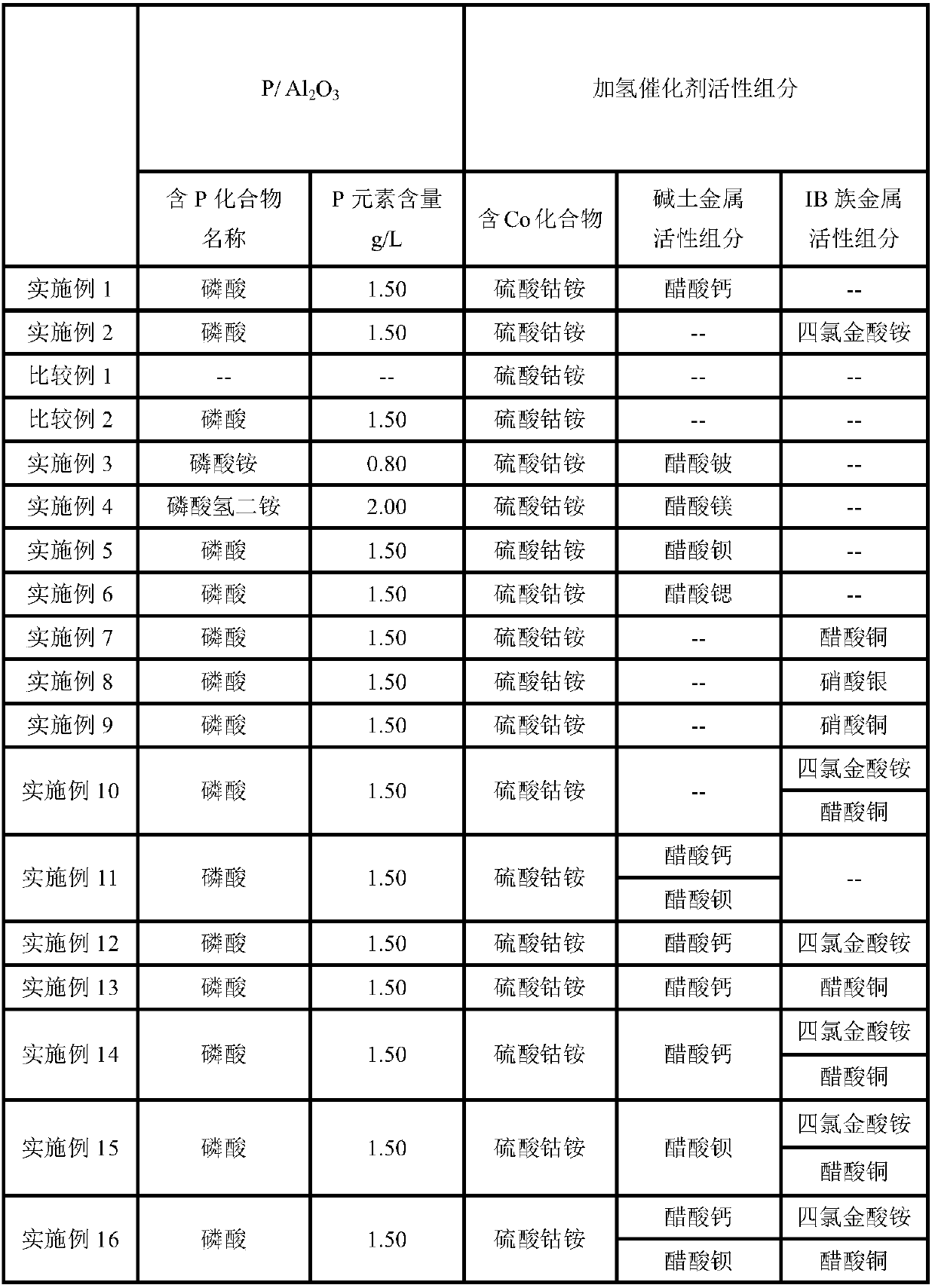
Embodiment 1
[0044] Modified carrier P / Al 2 o 3 Preparation of:
[0045] (1) Phosphoric acid (H 3 PO 4 ) aqueous solution 180ml impregnated in 1L diameter 6mm, pore volume is 0.92cm 3 / g, the specific surface area is 200cm 2 / g Al 2 o 3 , standing for 24 hours, and drying at 110° C. for 4 hours to obtain the carrier precursor I.
[0046] (2) Calcining the carrier precursor I at 630°C for 5h under a nitrogen gas atmosphere to obtain the modified carrier P / Al 2 o 3 .
[0047] The P content in the carrier was determined to be 1.50 g / L by ICP.
[0048] Preparation of hydrogenation catalyst:
[0049] (i) Cobalt ammonium sulfate containing 2.28g Co ((NH 4 ) 2 Co(SO 4 ) 2 ·6H 2 O) and calcium acetate containing 1.66g Ca (Ca(OAc) 2 ·H 2 O) 200ml of aqueous solution impregnated in P / Al 2 o 3 , to obtain catalyst precursor I;
[0050] (ii) drying at 110° C. for 4 hours to obtain the catalyst.
[0051] The Co content of the catalyst measured by ICP is 2.28g / L, and the Ca content...
Embodiment 2
[0056] Modified carrier P / Al 2 o 3 Preparation of:
[0057] (1) Phosphoric acid (H 3 PO 4 ) aqueous solution 180ml impregnated in 1L diameter 6mm, pore volume is 0.92cm 3 / g, the specific surface area is 200cm 2 / g Al 2 o 3 , standing for 24 hours, and drying at 110° C. for 4 hours to obtain the carrier precursor I.
[0058] (2) Calcining the carrier precursor I at 630°C for 5h under a nitrogen gas atmosphere to obtain the modified carrier P / Al 2 o 3 .
[0059] The P content in the carrier was determined to be 1.50 g / L by ICP.
[0060] Preparation of hydrogenation catalyst:
[0061] (i) Cobalt ammonium sulfate containing 2.28g Co ((NH 4 ) 2 Co(SO 4 ) 2 ·6H 2 O) and ammonium tetrachloroaurate ((NH 4 )AuCl 4 ·3H 2 O) 200ml of aqueous solution impregnated in P / Al 2 o 3 , to obtain catalyst precursor I;
[0062] (ii) drying at 110° C. for 4 hours to obtain the catalyst.
[0063] The Co content of the catalyst was measured by ICP to be 2.28g / L, and the Au con...
Embodiment 3
[0092] Modified carrier P / Al 2 o 3 Preparation of:
[0093] (1) Ammonium phosphate (NH 4 ) 3 PO 4 ) aqueous solution 180ml impregnated in 1L diameter 6mm, pore volume is 0.92cm 3 / g, the specific surface area is 200cm 2 / g Al 2 o 3 , standing for 24 hours, and drying at 110° C. for 4 hours to obtain the carrier precursor I.
[0094] (2) Calcining the carrier precursor I at 630°C for 5h under a nitrogen gas atmosphere to obtain the modified carrier P / Al 2 o 3 .
[0095] The P content in the carrier was determined by ICP to be 0.80 g / L.
[0096] Preparation of hydrogenation catalyst:
[0097] (i) Cobalt ammonium sulfate containing 2.28g Co ((NH 4 ) 2 Co(SO 4 ) 2 ·6H 2 O) and beryllium acetate (Be(OAc) containing 1.66g Be 2 ·H 2 O) be dissolved in hot water to obtain 200ml of impregnating solution and impregnate in P / Al 2 o 3 , to obtain catalyst precursor I;
[0098] (ii) drying at 110° C. for 4 hours to obtain the catalyst.
[0099] The Co content of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com