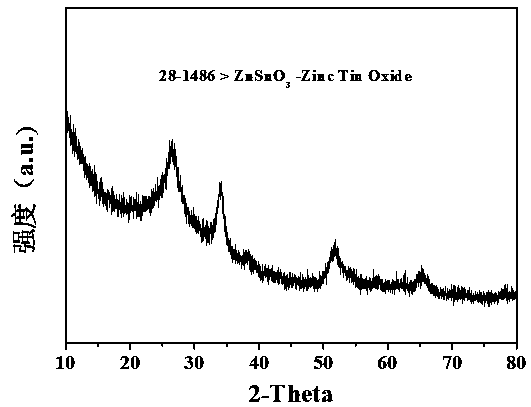
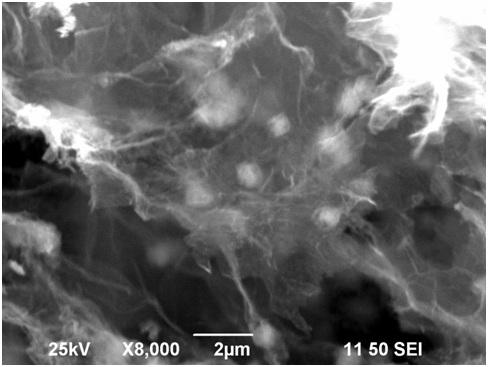
Preparation method of zinc stannate nanocube/graphene aerogel sunlight catalyst for degrading ciprofloxacin hydrochloride wastewater
A technology of ciprofloxacin hydrochloride and graphene aerogel, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve the problem of insufficient photocatalytic activity. Satisfaction, poor adsorption performance, etc., to achieve the effects of excellent chemical stability, high degradation efficiency, and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Step S1: Weigh 2.1036g SnCl with an analytical balance 4 ·5H 2 O in a beaker, add 30mL of ethanol, stir on a stirrer to make it completely dissolved to obtain solution A;
[0027] Step S2: Weigh 0.8178g ZnCl with an analytical balance 2 In the beaker, add 60mL of water, stir on the stirrer to completely dissolve to obtain solution B;
[0028] Step S3: After mixing solution A and solution B, place it on a magnetic stirrer and adjust the pH of the mixed system to 11.1 while stirring with a sodium hydroxide solution with a molar concentration of 2 mol / L, then filter the mixed solution, wash it with water, and dry it Get nano-cube zinc stannate overnight;
[0029] Step S4: using the improved Hummers' method to prepare graphene oxide with graphite powder as raw material, dispersing 0.6g graphene oxide in 60mL acetic acid aqueous solution, and obtaining a graphene oxide dispersion solution C with a concentration of 10 mg / mL after ultrasonication for 3 hours;
[0030] Step...
Embodiment 2
[0033] Step S1: Weigh 2.1036g SnCl with an analytical balance 4 ·5H 2 O in a beaker, add 30mL of ethanol, stir on a stirrer to make it completely dissolved to obtain solution A;
[0034] Step S2: Weigh 0.8178g ZnCl with an analytical balance 2 In the beaker, add 60mL of water, stir on the stirrer to completely dissolve to obtain solution B;
[0035] Step S3: After mixing solution A and solution B, place it on a magnetic stirrer and adjust the pH of the mixed system to 11.1 while stirring with a sodium hydroxide solution with a molar concentration of 2 mol / L, then filter the mixed solution, wash it with water, and dry it Get nano-cube zinc stannate overnight;
[0036] Step S4: using the improved Hummers' method to prepare graphene oxide with graphite powder as raw material, dispersing 0.6g graphene oxide in 60mL acetic acid aqueous solution, and obtaining a graphene oxide dispersion solution C with a concentration of 10 mg / mL after ultrasonication for 3 hours;
[0037] Step S...
Embodiment 3
[0040] Step S1: Weigh 2.1036g SnCl with an analytical balance 4 ·5H 2 O in a beaker, add 30mL of ethanol, stir on a stirrer to make it completely dissolved to obtain solution A;
[0041] Step S2: Weigh 0.8178g ZnCl with an analytical balance 2 In the beaker, add 60mL of water, stir on the stirrer to completely dissolve to obtain solution B;
[0042] Step S3: After mixing solution A and solution B, place it on a magnetic stirrer and adjust the pH of the mixed system to 11.1 while stirring with a sodium hydroxide solution with a molar concentration of 2 mol / L, then filter the mixed solution, wash it with water, and dry it Get nano-cube zinc stannate overnight;
[0043] Step S4: using the improved Hummers' method to prepare graphene oxide with graphite powder as raw material, dispersing 0.6g graphene oxide in 60mL acetic acid aqueous solution, and obtaining a graphene oxide dispersion solution C with a concentration of 10 mg / mL after ultrasonication for 3 hours;
[0044] Step...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com