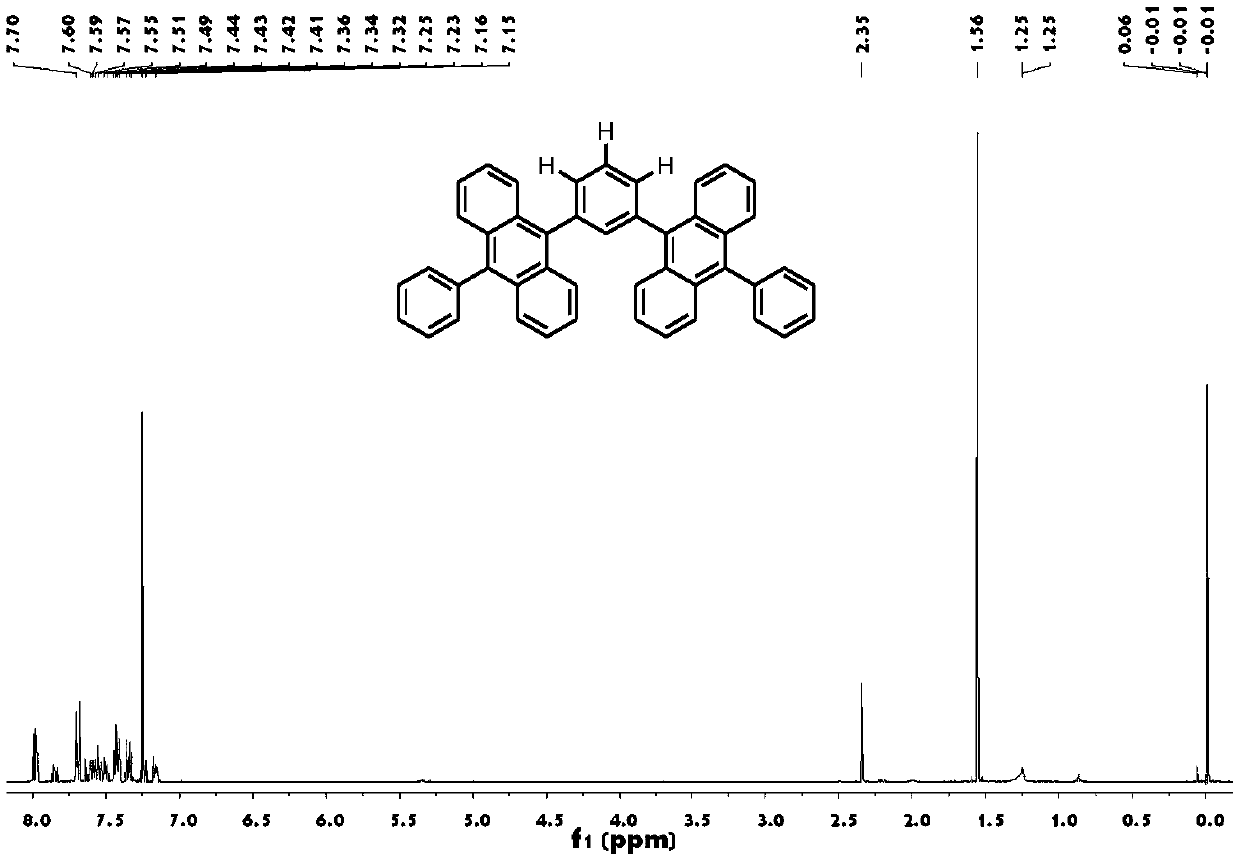
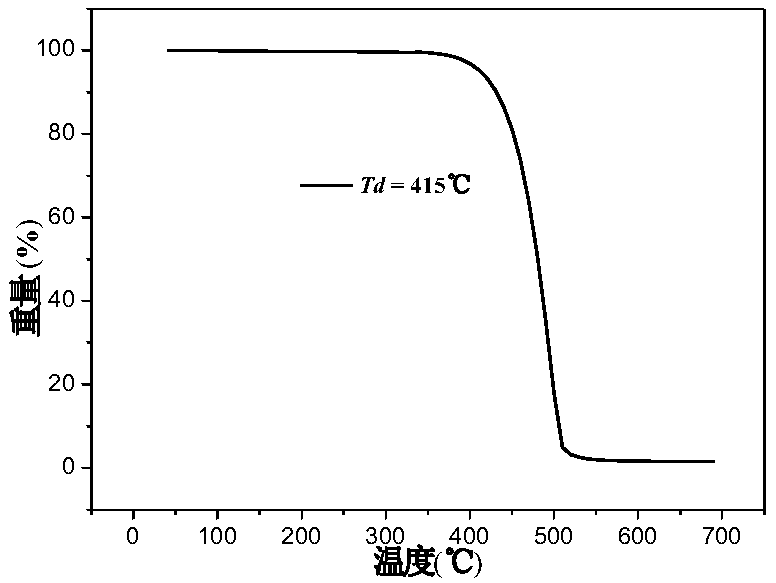
Organic blue fluorescent material based on dianthracene as well as preparation method and application of organic blue fluorescent material
A blue fluorescent, organic technology, applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of reducing the efficiency and stability of OLED devices, unable to meet commercial production, and poor film formation, and achieve the realization of The effect of dark blue emission, suppression of π-π accumulation, and reduction of production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] ①Add 2.60g of 9-bromoanthracene, 1.83g of phenylboronic acid, 13.82g of potassium carbonate (add 30mL of distilled water to make a 2.0M solution), 100mL of toluene, and 30mL of ethanol into the reaction flask, then add 0.58g of tetrakis(triphenylphosphine )palladium. Then vacuumize the system, and reflux at 100° C. for 12 hours under the protection of nitrogen. After the reaction, the product was obtained by toluene extraction, rotary evaporation, column chromatography (eluent: n-hexane), and recrystallization (n-hexane / toluene=4:1). Yield 88%.
[0054] ②Bromination of 9-benzoanthracene: Add 2.15g of 9-benzoanthracene, 100mL of DMF, and 1.80g of NBS into the reaction flask, then vacuumize the system, and react at 85°C for 1 hour under the protection of nitrogen. After the reaction, the product was washed with methanol and suction filtered to obtain the product 9-bromo-10-benzanthracene. Yield 89%.
[0055] ③9-Bromo-10-Benzanthracene Boronate: Add 1.40g of 9-Bromo-10...
Embodiment 2
[0058] ①Add 3.50g of 9-bromoanthracene, 2.52g of phenylboronic acid, 18.82g of potassium carbonate (add 45mL of water to form a solution), 136mL of toluene, and 45mL of ethanol into the reaction flask, and finally add 0.82g of tetrakis(triphenylphosphine) palladium. The system was evacuated and refluxed at 105° C. for 18 hours under nitrogen protection. After the reaction, the product was obtained by toluene extraction, rotary evaporation, column chromatography (eluent: n-hexane), and recrystallization (n-hexane / toluene=4:1). Yield 86%. ②9-Benzanthracene bromination: Add 3.50 g of 9-Benzanthracene, 130 mL of DMF, and 2.94 g of NBS into the reaction flask, then vacuumize the system, and react at 88°C for 1.5 h under the protection of nitrogen. After the reaction, the product was washed with methanol and filtered with suction to obtain the product 9-bromo-10-benzanthracene with a yield of 87%. ③Boronization of 9-bromo-10-benzanthracene: Add 2 g of 9-bromo-10-benzanthracene, 1...
Embodiment 3
[0060]①Add 5.14g of 9-bromoanthracene, 3.66g of phenylboronic acid, 27.64g of potassium carbonate (add 60mL of water to form a solution), 200mL of toluene, and 60mL of ethanol into the reaction flask, and finally add 1.16g of tetrakis(triphenylphosphine)palladium . Then the system was evacuated and refluxed at 110° C. for 24 hours under the protection of argon. After the reaction, the product was obtained by toluene extraction, rotary evaporation, column chromatography (eluent: n-hexane), and recrystallization (n-hexane / toluene=4:1). Yield 86%. ②9-Benzanthracene bromination: Add 4.29g of 9-benzanthracene, 200mL of DMF, and 3.6g of NBS into the reaction flask, then vacuumize the system, and react at 90°C for 2 hours under the protection of argon. After the reaction was completed, the product was washed with methanol and suction filtered to obtain the product 9-bromo-10-benzanthracene with a yield of 77%. ③9-Bromo-10-benzoanthracene boronate: Add 2.80g of 9-bromo-10-benzoanth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com