A kind of antibacterial polypeptide hf-18 and its preparation method and application
A HF-18, antibacterial peptide technology, applied in the direction of antibacterial drugs, peptides, peptide sources, etc., can solve the problems of increased clinical use of antibiotics, slow and difficult discovery of antibacterial drugs, etc., to relieve inflammation, and design and preparation methods are simple and convenient. , the effect of improving antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of antibacterial polypeptide HF-18:
[0046](1) Using the polypeptide HFIAP-1 as a template, intercept the region where its sequence is conserved and the α-helix is highly concentrated, and intercept from the first position of the N-terminus to reduce the number of polypeptide amino acids from 37 to 18, reducing the cost of synthesis;
[0047] (2) Using the truncated 18 amino acids as a template, replace histidine at position 12 with positively charged lysine and glycine at position 14, which is not conducive to α-helix formation, with hydrophobic phenylalanine , the overall positive charge was increased from 7 to 8, and the hydrophobicity value was increased from 0.207 to 0.244, thereby obtaining the amino acid sequence of the polypeptide HF-18.
[0048] (3) Polypeptide HF-18 synthesized by solid phase synthesis
[0049] The amino acid sequence of the prepared HF-18 is: glycine-phenylalanine-phenylalanine-lysine-lysine-alanine-tryptophan-arginine-lysine-...
Embodiment 2
[0053] Purity determination (HPLC method) and mass spectrometry analysis results of polypeptide HF-18:
[0054] Example 1 Polypeptide HF-18 was synthesized and purified to obtain a finished product, which was identified by high performance liquid chromatography and mass spectrometry.
[0055] Liquid chromatography analysis conditions: C18 chromatographic column (4.6×250mm, 5 μm); mobile phase A is acetonitrile solution containing 0.1% trifluoroacetic acid, and mobile phase B is pure water containing 0.1% trifluoroacetic acid. The detection wavelength is 220nm; the flow rate is 1.0ml / min; the injection volume is 20μl, and gradient elution is performed. See Table 1 for gradient elution conditions.
[0056] Table 2 gradient elution conditions
[0057]
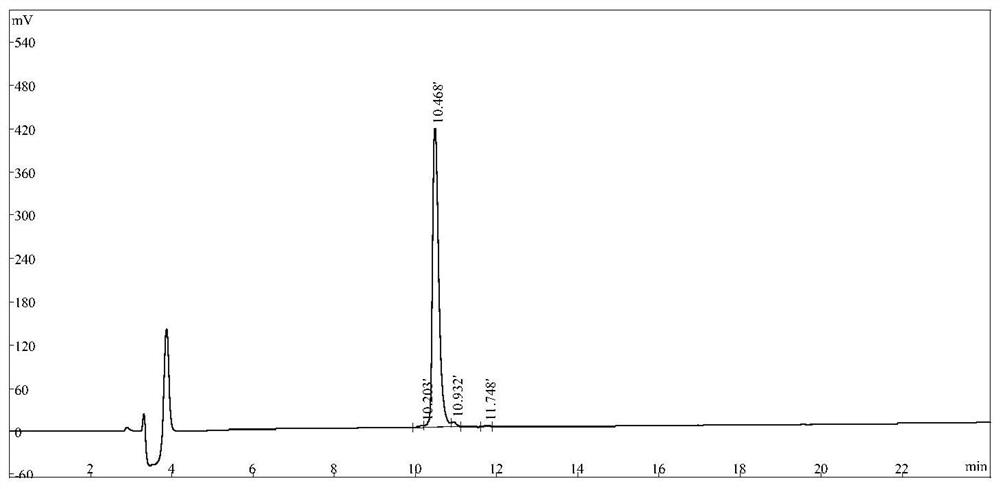
[0058] Depend on figure 1 It can be seen that the purity of the polypeptide HF-18 prepared in Example 1 of the present invention is greater than 98%.
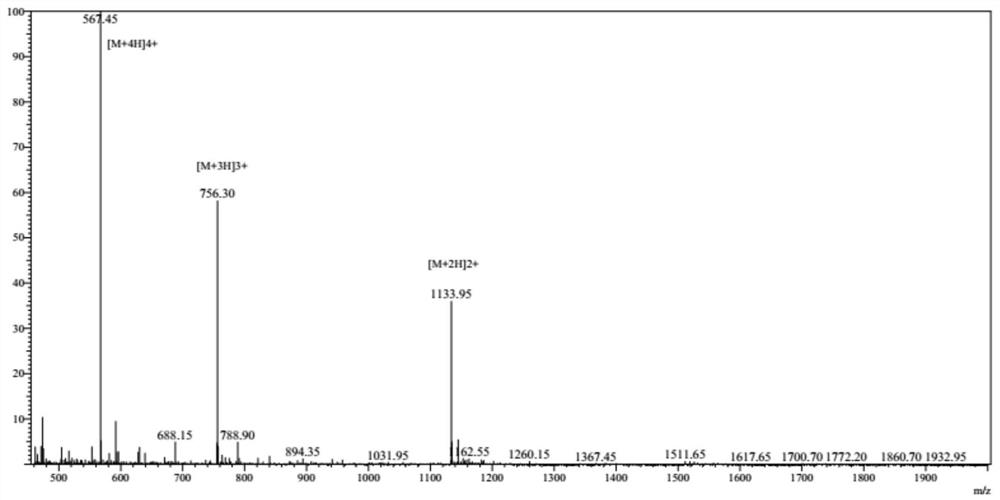
[0059] Depend on figure 2 To determine, the molecular weight of HF-18...
Embodiment 3
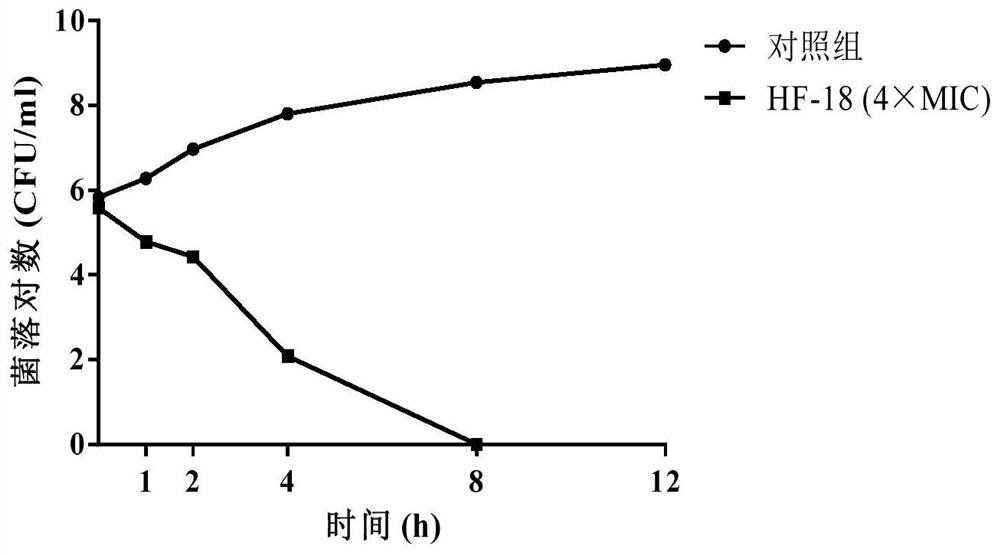
[0061] Determination of antibacterial activity of polypeptide HF-18 of the present invention in vitro
[0062] (1) Recovery and activation of strains
[0063] The experimental strains frozen at -20°C were transferred from the glycerol tube to the corresponding agar slant medium (bacteria to nutrient agar slant, fungi to Sabouraud dextrose agar slant). Bacteria were cultured at 37°C for 24 hours, fungi were cultured at 25°C for 48 hours, and placed in a refrigerator at 4°C for later use.
[0064] (2) Preparation of bacteria solution
[0065] Inoculate a little of the bacteria in the slant, transfer to 2ml of the corresponding liquid medium, culture at 37°C or 25°C for 8 hours, and dilute to 10% with liquid medium 5 CFU / ml bacterial suspension, for later use.
[0066] (3) Drug preparation
[0067] The polypeptide HF-18 prepared in Example 1 and HFIAP-1 were weighed and dissolved in physiological saline respectively, and both were prepared into a mother solution of 1024 μg / ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com