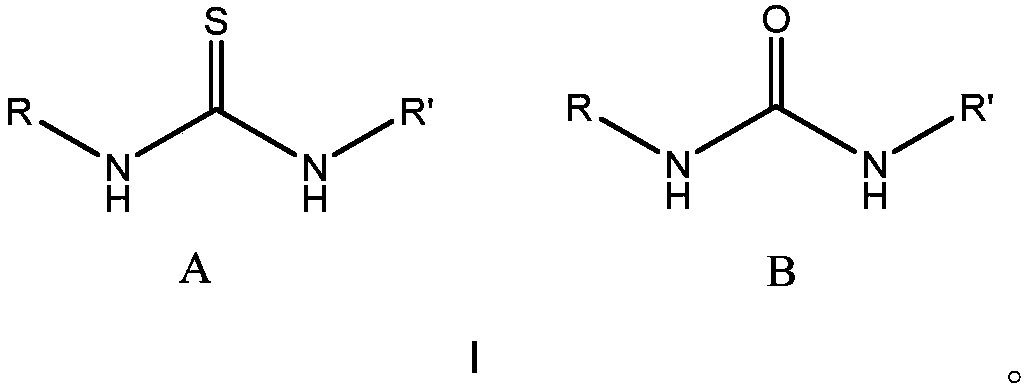
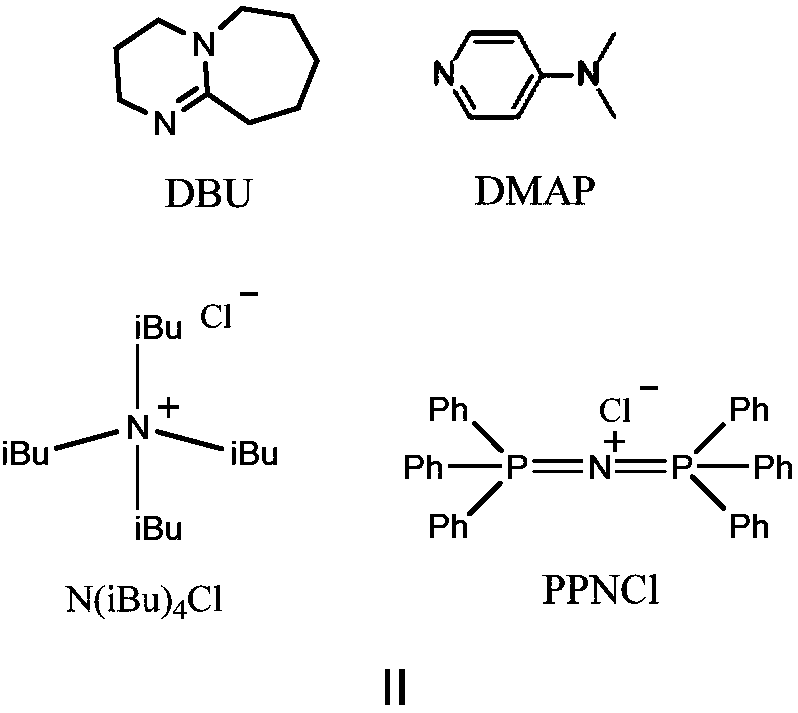
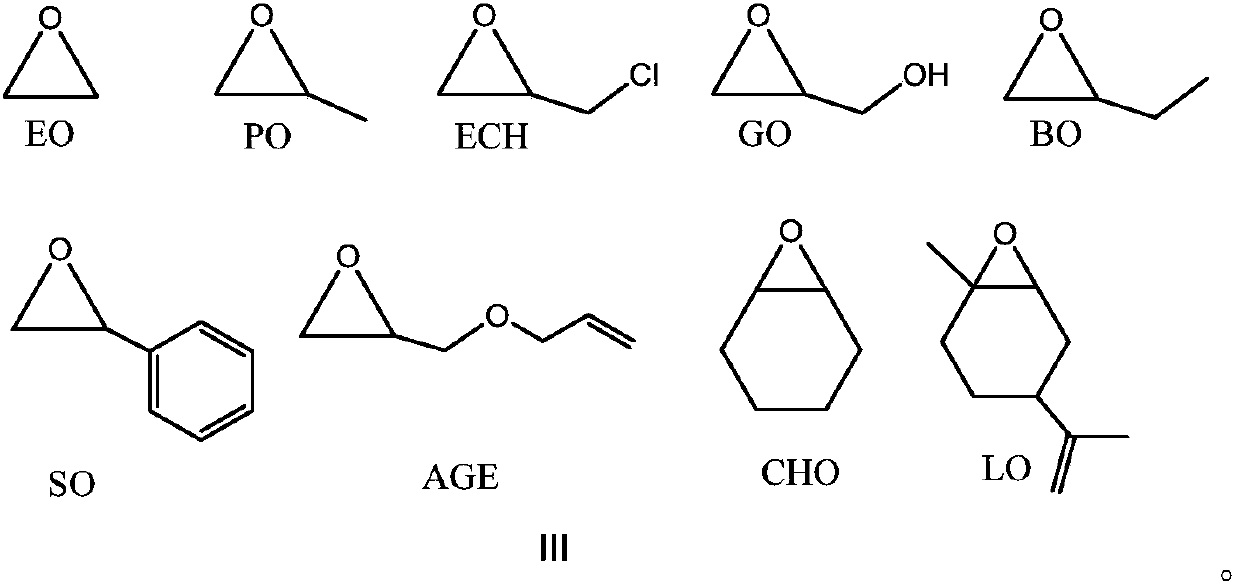
Method for preparing polyester from (thio)urea/organic alkali catalyzed epoxides and cyclic anhydrides by ring-opening copolymerization
A technology for catalyzing rings and cyclic acid anhydrides with organic bases, applied in the field of polymer materials, can solve problems such as increasing production costs, and achieve the effects of low price, industrialization and application, simple preparation and economy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, urea and DBU are used for the ring-opening copolymerization of CHO and PA
[0030] In an anhydrous and oxygen-free environment, add 0.05mmol urea, 0.05mmol DBU, 5mmol PA and 25mmol CHO to the flask in turn, stir evenly at room temperature, place it in a set 110°C constant temperature reaction bath for 20min, and take samples for NMR analysis . The product was dissolved in dichloromethane, and the polymer was obtained by precipitation with methanol, which was vacuum-dried by suction and then tested for molecular weight. PA conversion rate: 48%, polyester selectivity: 95%, monomer amount converted per unit catalyst per unit time (TOF): 144, molecular weight (Mn): 4.7KDa, molecular weight distribution: PDI=1.27.
Embodiment 2
[0031] Embodiment 2, urea and DBU and benzyl alcohol are used for the ring-opening copolymerization of CHO and PA
[0032] In an anhydrous and oxygen-free environment, add 0.05mmol urea, 0.05mmol DBU, 0.05mmol benzyl alcohol, 5mmol PA and 25mmol CHO to the flask in turn, stir evenly at room temperature, and place it in a set 110°C constant temperature reaction bath for 20min. , sampled for NMR analysis. The product was dissolved in dichloromethane, and the polymer was obtained by precipitation with methanol, which was vacuum-dried by suction and then tested for molecular weight. PA conversion rate: 52%, polyester selectivity: 96%, monomer amount converted per unit catalyst unit time (TOF): 156, molecular weight (Mn): 2.9KDa, molecular weight distribution: PDI=1.25.
Embodiment 3
[0033] Embodiment 3, urea and DMAP are used for the ring-opening copolymerization of CHO and PA
[0034] In an anhydrous and oxygen-free environment, add 0.05mmol urea, 0.05mmol DMAP, 5mmol PA and 25mmol CHO to the flask in turn, stir evenly at room temperature, place it in a set 110°C constant temperature reaction bath for 40min, and take samples for NMR analysis . The product was dissolved in dichloromethane, and the polymer was obtained by precipitation with methanol, which was vacuum-dried by suction and then tested for molecular weight. PA conversion rate: 29%, polyester selectivity: 95%, monomer amount converted per unit catalyst per unit time (TOF): 44, molecular weight (Mn): 3.1KDa, molecular weight distribution: PDI=1.18.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com