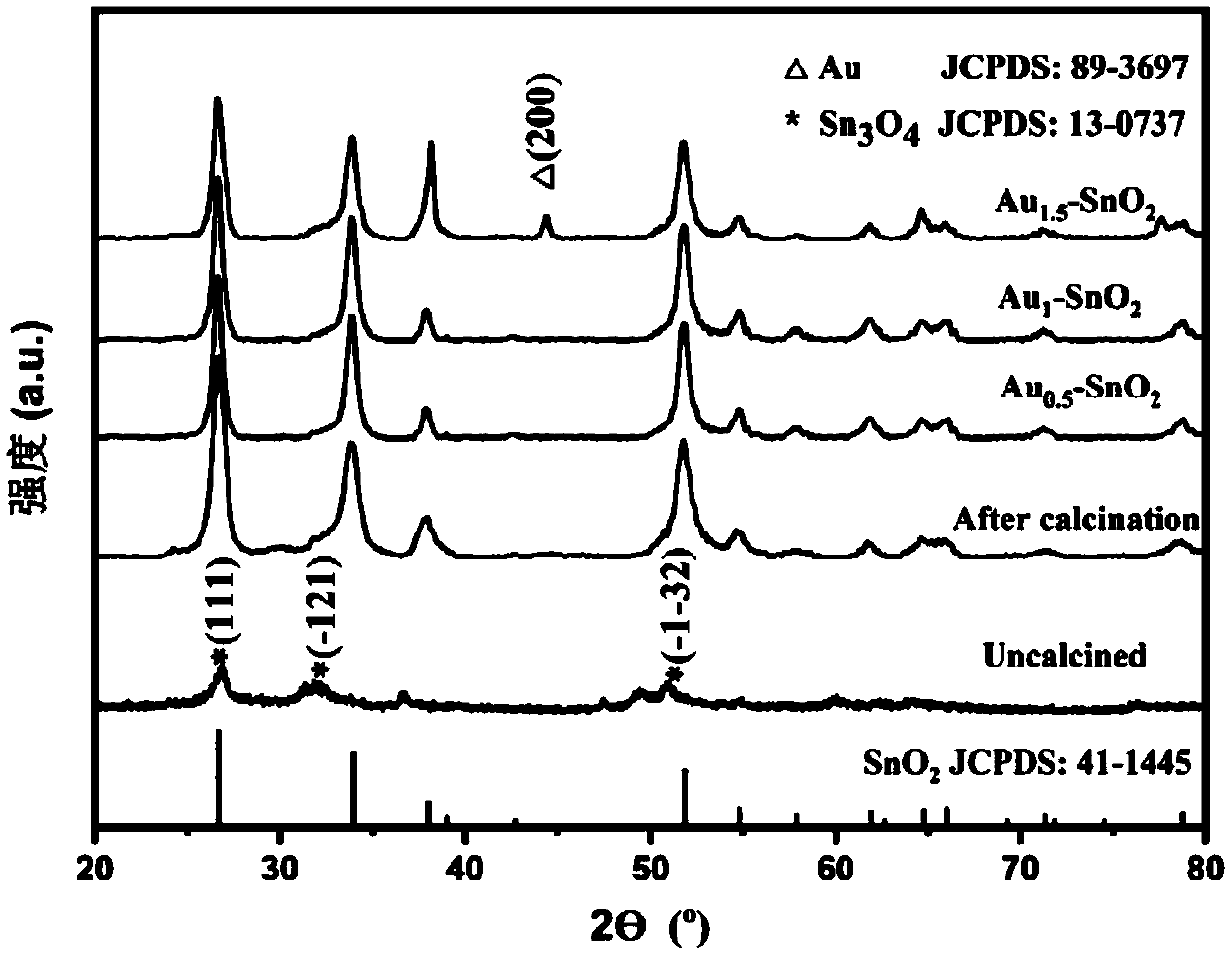
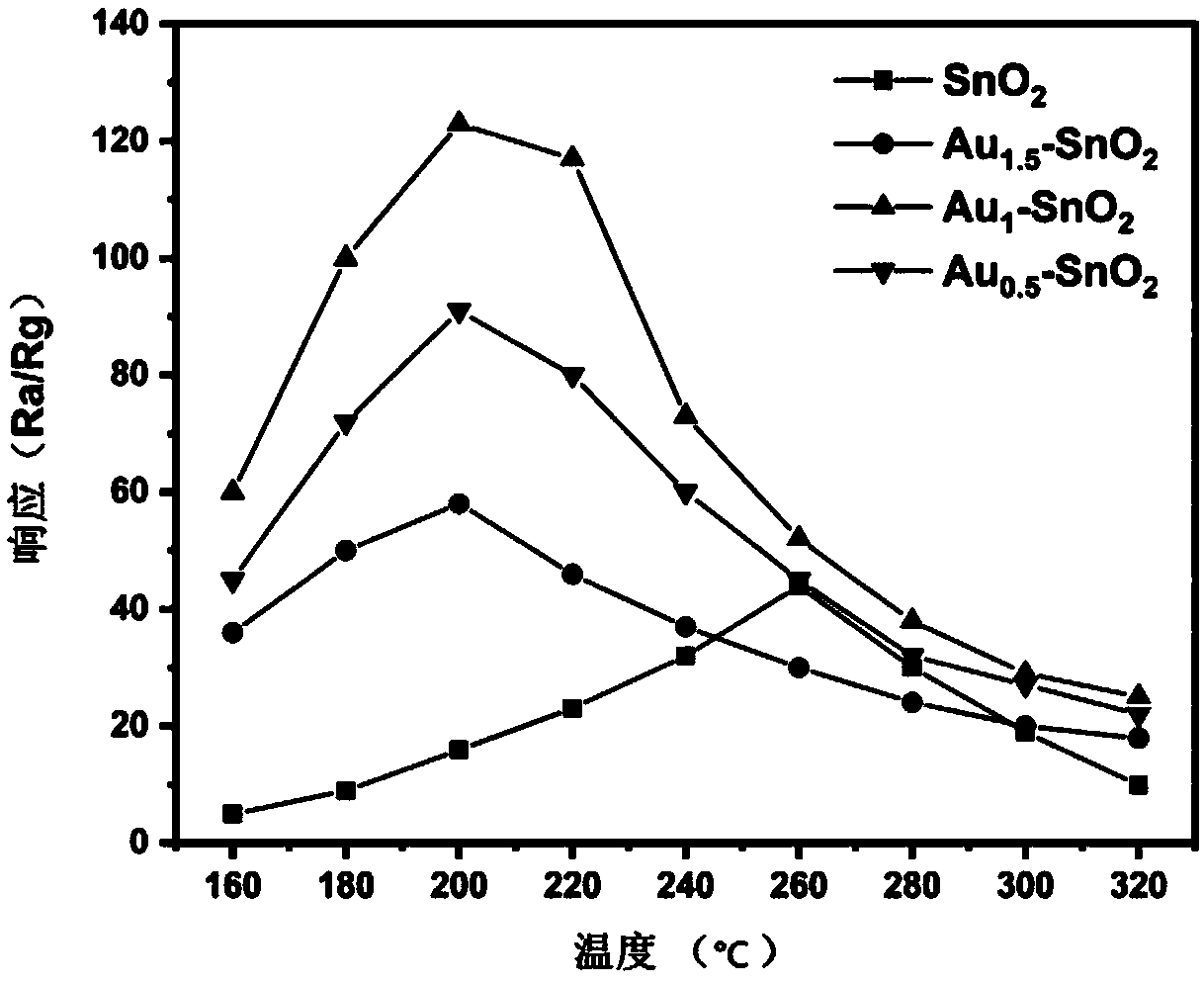
Self-reduction preparation method of gold nanoparticle loaded tin dioxide nanoflower gas-sensing material
A technology of gold nanoparticles and tin dioxide, applied in chemical instruments and methods, alkali metal oxides/hydroxides, nanotechnology, etc., can solve problems such as material pollution and difficult experiments, simplify the experimental process, avoid Effects of contamination, increased sensitivity and response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Dissolve 10mmol of sodium citrate in 20ml of deionized water, and add 0.12g of sodium hydroxide. Dissolve 5mmol of stannous chloride in 20ml of ethanol. After the two solutions were completely dissolved, the ethanol solution was added to the aqueous solution and stirred at room temperature for 1 h.
[0026] (2) The solution obtained in step (1) was transferred to a 50ml reactor, reacted at 180°C for 12h, and then cooled naturally to room temperature.
[0027] (3) The product obtained in step (2) was centrifuged and washed several times with deionized water and ethanol, and dried at 60° C. for 12 hours to obtain tin trioxide nanoflowers.
[0028] (4) Ultrasonically disperse 0.1 g of the powder obtained in step (3) in 20 ml of deionized water to obtain a suspension.
[0029] (5) Add chloroauric acid solution (concentration: 10 mg / ml) to the suspension obtained in step (4), stir at room temperature for 1 h, and control the atomic ratio of gold and tin to 1:100.
[0...
Embodiment 2
[0034] (1) Dissolve 10mmol of sodium citrate in 20ml of deionized water, and add 0.14g of sodium hydroxide. Dissolve 5mmol of stannous chloride in 20ml of ethanol. After the two solutions were completely dissolved, the ethanol solution was added to the aqueous solution and stirred at room temperature for 1 h.
[0035] (2) The solution obtained in step (1) was transferred to a 50ml reactor, reacted at 180°C for 12h, and then cooled naturally to room temperature.
[0036] (3) The product obtained in step (2) was centrifuged and washed several times with deionized water and ethanol, and dried at 60° C. for 12 hours to obtain tin trioxide nanoflowers.
[0037] (4) Ultrasonically disperse 0.1 g of the powder obtained in step (3) in 20 ml of deionized water to obtain a suspension.
[0038] (5) Add chloroauric acid solution (concentration: 10 mg / ml) to the suspension obtained in step (4), stir at room temperature for 1 h, and control the atomic ratio of gold and tin to 0.5:100.
...
Embodiment 3
[0042] (1) Dissolve 10mmol of sodium citrate in 20ml of deionized water, and add 0.16g of sodium hydroxide. Dissolve 5mmol of stannous chloride in 20ml of ethanol. After the two solutions were completely dissolved, the ethanol solution was added to the aqueous solution and stirred at room temperature for 1 h.
[0043] (2) The solution obtained in step (1) was transferred to a 50ml reactor, reacted at 180°C for 12h, and then cooled naturally to room temperature.
[0044] (3) The product obtained in step (2) was centrifuged and washed several times with deionized water and ethanol, and dried at 60° C. for 12 hours to obtain tin trioxide nanoflowers.
[0045] (4) Ultrasonically disperse 0.1 g of the powder obtained in step (3) in 20 ml of deionized water to obtain a suspension.
[0046] (5) Add chloroauric acid solution (concentration: 10 mg / ml) to the suspension obtained in step (4), stir at room temperature for 1 h, and control the atomic ratio of gold and tin to 1.5:100.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com