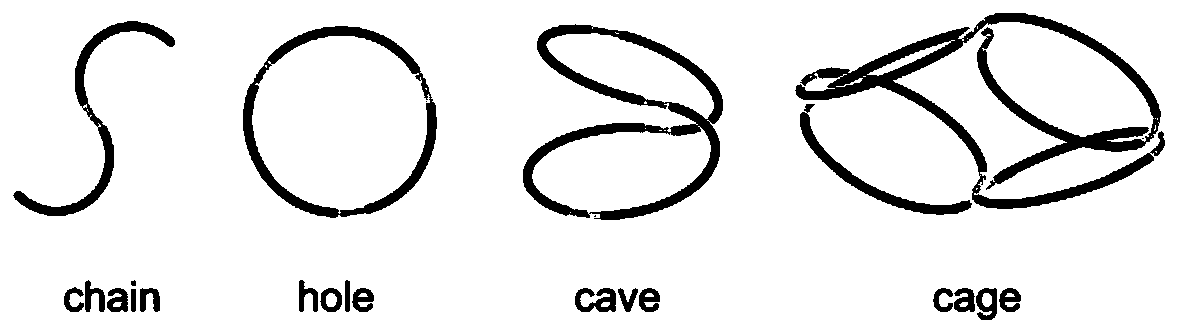
Framework molecules based on folding and assembling of cyclic polymers and preparation method thereof
A framework and ring polymerization technology, applied in the field of supramolecular chemistry, can solve problems such as poor recognition ability of guest molecules, difficulty in exchanging inside and outside molecular pores, high cost of separation and purification, and achieve excellent performance, economical and easy to obtain raw materials, and low cost of separation and purification Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0048] The present invention will be described in further detail below in conjunction with specific examples, but the present invention is not limited thereto.
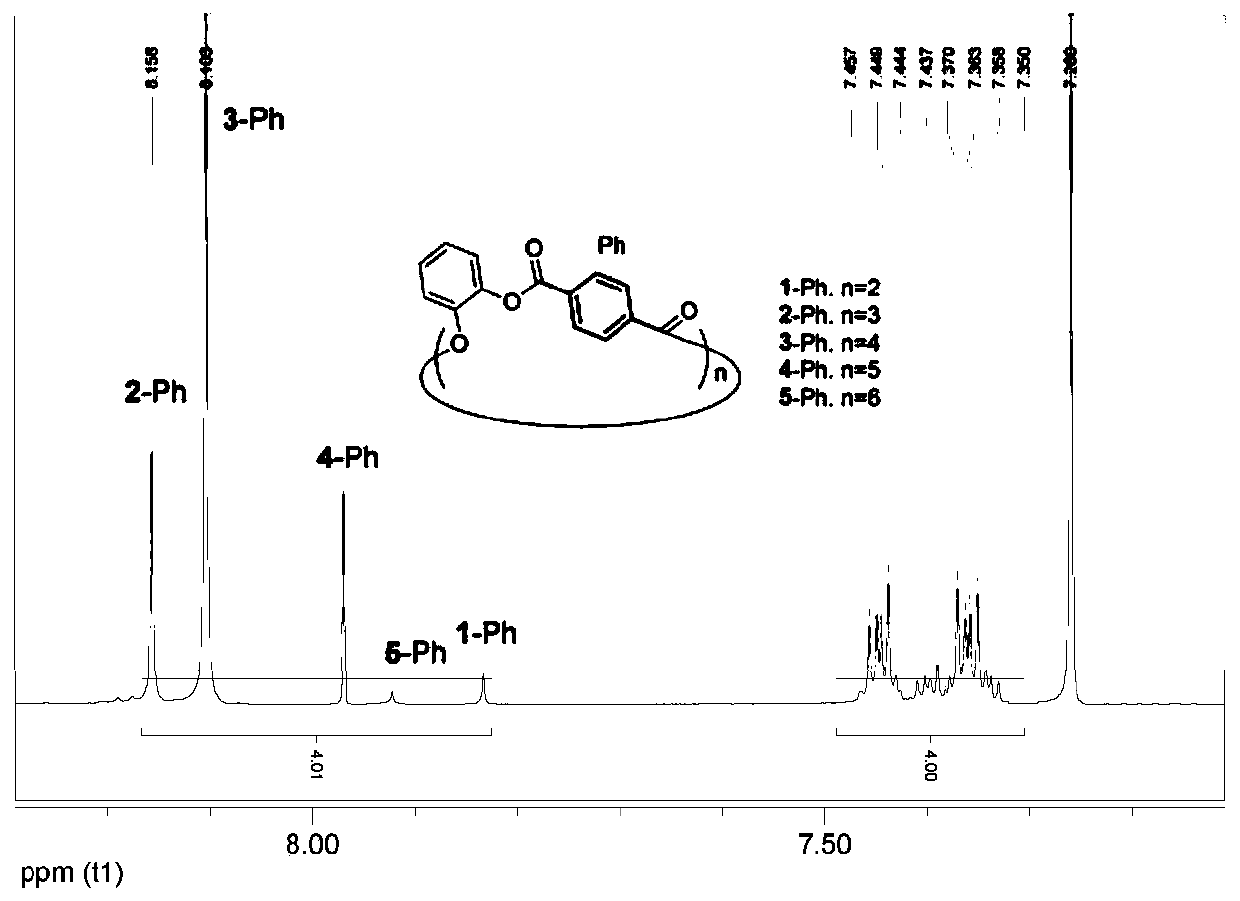
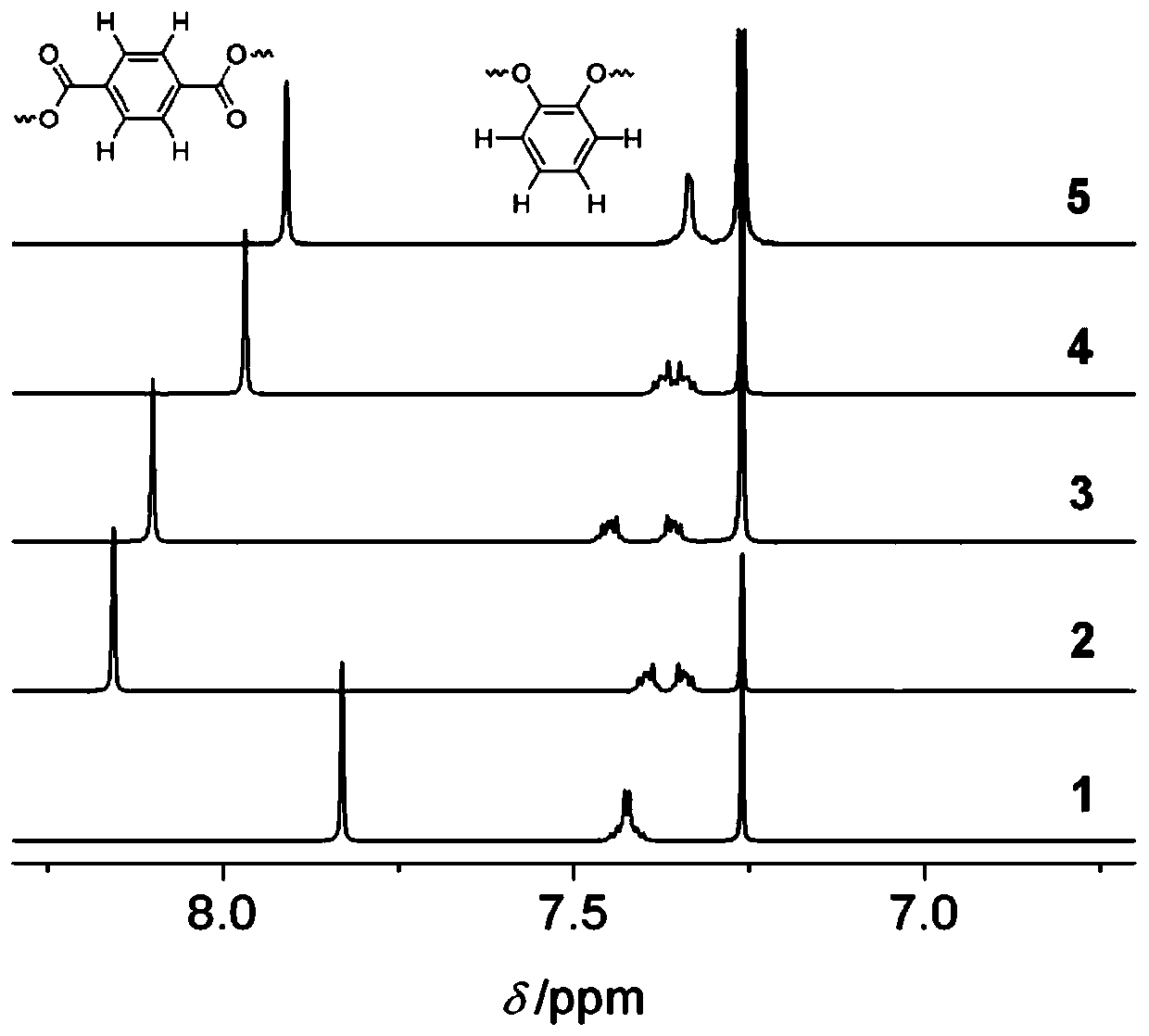
[0049]The following examples are based on the synthesis of phenyl 1,4-bridged cyclic polyester series, and the preparation, separation, characterization and structural characteristics of phenylene terephthalate (1-Ph to 5-Ph) are all described through the examples. Illustrate, and further describe the present invention by illustration of accompanying drawing. Figure 2 to Figure 15 Evidences such as nuclear magnetic resonance spectrum, mass spectrum, single crystal diffraction and theoretical calculation results of this series of cyclic polyesters are provided.
[0050] Phenyl 1,4-bridged ring polyester series, 1-Ph, 2-Ph, 3-Ph, 4-Ph and 5-Ph, the compound structure formula is shown in formula (1):
[0051]
[0052] Its compound synthetic train of thought route is as shown in formula (2):
[0053]
[0054] As ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com