Integral catalyst for preparing chlorine by catalytic oxidation of hydrogen chloride and application of integral catalyst
A monolithic catalyst, catalytic oxidation technology, applied in the preparation of chlorides, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., can solve the problem of low overall efficiency, high cost, and clogging of the reactor. and other problems, to achieve the effect of inhibiting heat accumulation, reducing manufacturing difficulty and manufacturing cost, and preventing heat accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
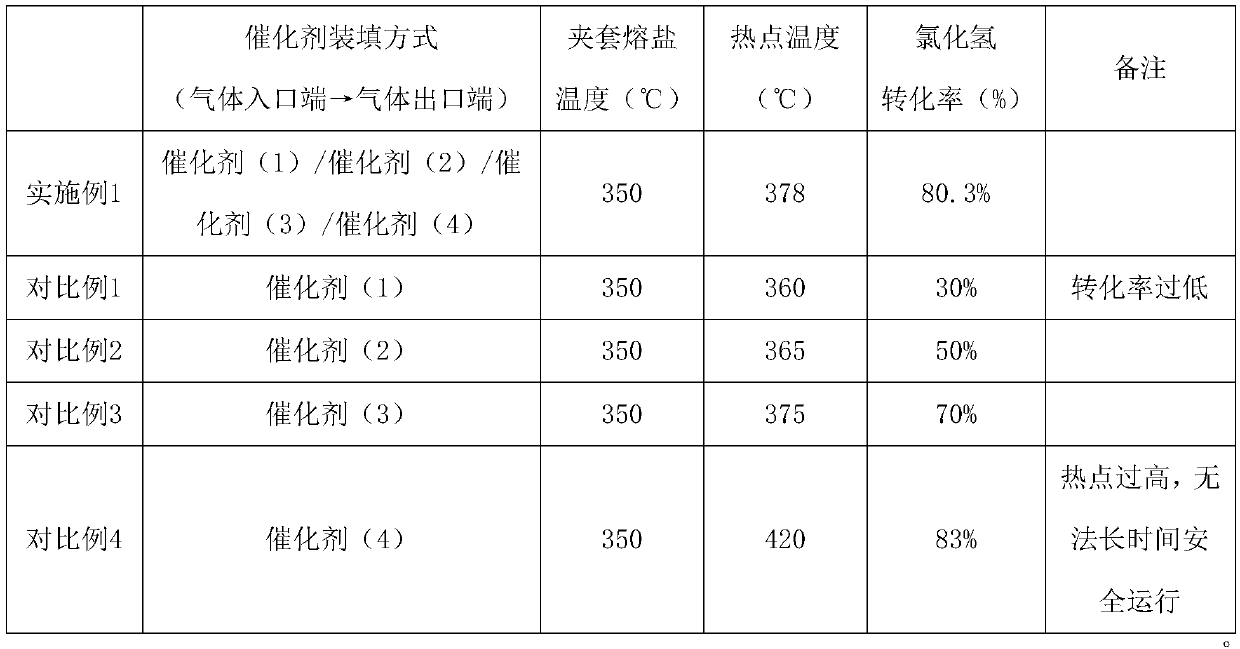
Examples
Embodiment 1
[0038] 9.16kgCuCl 2 2H 2 O, 1.24kg VCl 3 , 4.76kgKCl, 4.54kgCeCl 3 ·7H 2 O drop into 86.53kg of pure water to make a solution; drop 90kg of silicon dioxide powder and 4.5kg of binder into the above solution to make a slurry; put the above slurry into an extruder, and extrude a diameter of 40mm Honeycomb-shaped cylindrical strips were cut into 100 cm lengths; the above-mentioned cylindrical strips were put into a blast oven at 100°C for 12 hours, and then transferred to a muffle furnace at 450°C for 6 hours to obtain monolithic catalyst (1). The activity is 0.29kgCl 2 / (kgcat h);
[0039] Wherein the mass percent of each component is:
[0040] Transition metals (as oxides): 5%
[0041] Alkali metal (as oxide): 3%
[0042] Rare earth metals (calculated as oxides): 2%
[0043] Carrier: margin
[0044] The transition metals are Cu and V, and Cu:V (in terms of oxide mass) is 6:1;
[0045] The alkali metal is potassium;
[0046] The rare earth metal is Ce;
Embodiment 2
[0048] 14.25kgCuCl 2 2H 2 O, 2.31kg VCl 3 , 6.34kgKCl, 6.84kgLaCl 3 ·7H 2 O was put into 79.68kg of pure water to form a solution; 85kg of activated alumina powder and 4.25kg of binder were added to the above solution to make a slurry; the above slurry was put into an extruder, and the extrusion diameter was 40mm The briquette-shaped cylindrical strips are cut into 50 cm lengths; the above-mentioned cylindrical strips are put into a 110° C. blast oven for drying for 11 hours, and then transferred to a 460° C. muffle furnace for roasting for 5 hours to obtain monolithic catalysts (2); the activity is 0.49kgCl 2 / (kgcat h);
[0049] Transition metals (as oxides): 8%
[0050] Alkali metal (as oxide): 4%
[0051] Rare earth metals (calculated as oxides): 3%
[0052] Carrier: margin
[0053] The transition metals are Cu and V, and Cu:V (in terms of oxide mass) is 5:1;
[0054] The alkali metal is potassium;
[0055] The rare earth metal is La;
Embodiment 3
[0057] 21.38kgCuCl 2 2H 2 O, 3.46kg VCl 3 , 6.34kgKCl, 9.05kgPrCl 3 ·7H 2O drop into 72.45kg of pure water to make a solution; drop 40kg of silicon dioxide powder, 40kg of activated alumina powder and 4kg of binding agent into the above solution to make a slurry; put the above slurry into an extruder, extrude A honeycomb briquette-shaped cylindrical strip with a diameter of 40 mm is cut into a length of 50 cm; the above-mentioned cylindrical strip is dropped into a 120° C. blast oven for drying for 10 hours, and then transferred to a 480° C. muffle furnace for roasting for 5 hours to obtain a monolithic catalyst ( 3). The activity is 0.68kgCl 2 / (kgcat h);
[0058] Wherein the mass percent of each component is:
[0059] Transition metals (as oxides): 12%
[0060] Alkali metal (as oxide): 4%
[0061] Rare earth metals (calculated as oxides): 4%
[0062] Carrier: margin
[0063] The transition metals are Cu and V, and Cu:V (in terms of oxide mass) is 5:1;
[0064] Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com