Biological preparation method of gamma-aminobutyric acid
A technology for the preparation of aminobutyric acid and biology, applied in the field of biochemistry, can solve the problems of complex purification of γ-aminobutyric acid, high price of L-glutamic acid, and low price of sodium glutamate, and achieve complete conversion of raw materials, Environmentally friendly process and simple post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
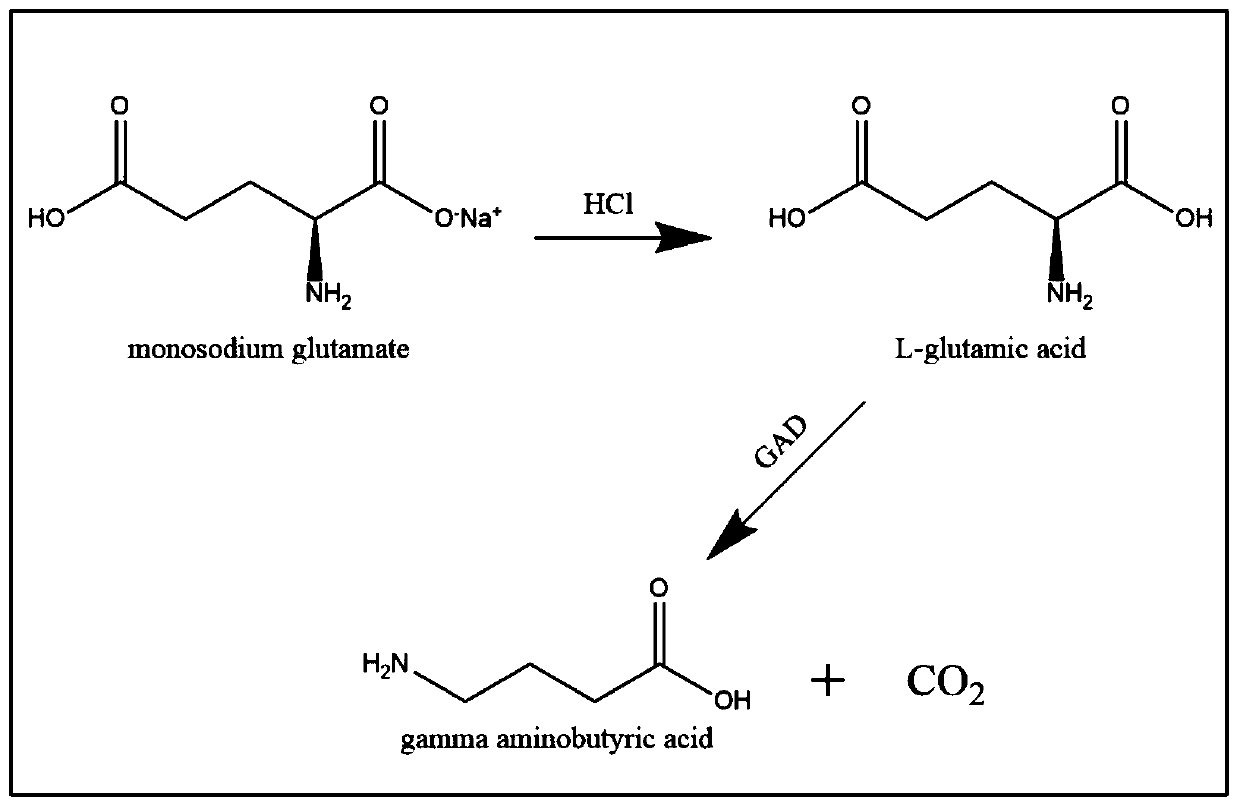
[0029] Embodiment 1 prepares L-glutamic acid with monosodium glutamate
[0030] Dissolve 30g of monosodium glutamate in 80mL of water, add 14mL of concentrated hydrochloric acid to convert the monosodium glutamate into L-glutamic acid and precipitate it from the water, wash the filtered L-glutamic acid with water for several times until the pH of the washed liquid is greater than 3 Stop washing when the time comes, collect L-glutamic acid, and use it in the reaction after drying.
Embodiment 2
[0031] Example 2 Preparation of glutamic acid decarboxylase whole cell
[0032] Inoculate the Gad-pET28a recombinant Escherichia coli engineering strain into SOB liquid medium, which contains 50mg / L of kanamycin; after inoculation, shake the flask on a shaker at 37°C and 200rpm, when the OD of the bacteria 600 When it reached 0.6, it was transferred to a 5L fermenter containing 2L SOB medium, and the inoculation ratio was 10%. The fermentation temperature in the early stage of the reaction is 37°C, the enzyme production temperature in the later stage is 25°C, the stirring speed is 400rpm, the aeration ratio and tank pressure are 2VVM (cubic meter / (cubic meter*min)) and 0.05MPa respectively; Sampling was taken to monitor the cell mass and determine the enzyme activity. When the cell mass and enzyme activity remained stable, the fermentation was stopped, and the resting cells were collected by centrifugation at 4°C (6000rpm, 5min), and the wet cell mass reached 60g / L.
Embodiment 3
[0033] Embodiment 3 prepares gamma-aminobutyric acid
[0034] 500mL reaction system to prepare the compound γ-aminobutyric acid: add 500mL water to a 1L reaction kettle, add 13g whole cells of glutamic acid decarboxylase, add pyridoxal phosphate with a final concentration of 0.5mM, and add L-glutamic acid at a time 100g, react at 35-40°C, 220rpm for 7 hours, use thin-layer chromatography to detect the progress of the end point of the reaction, add 100g of L-glutamic acid when the substrate is reacted, repeat in sequence, and add L-glutamic acid in total After 350 g and complete reaction, the supernatant was collected by centrifugation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com