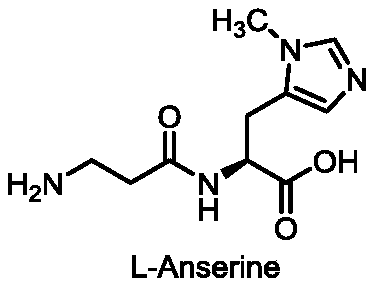
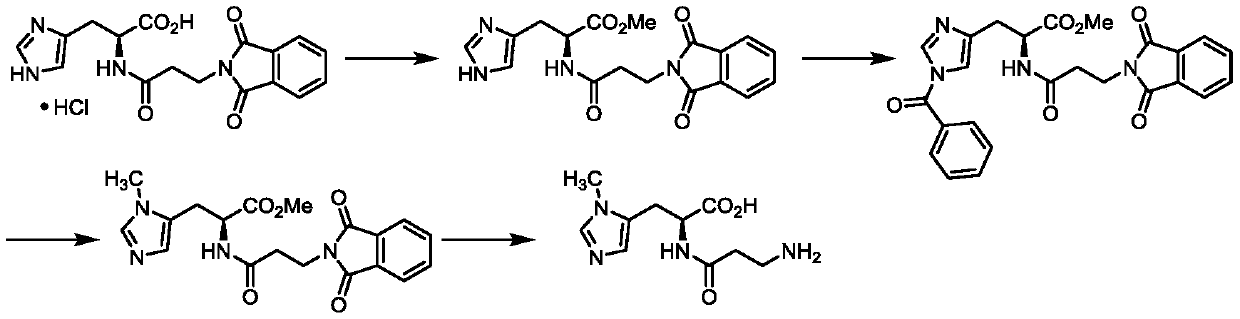
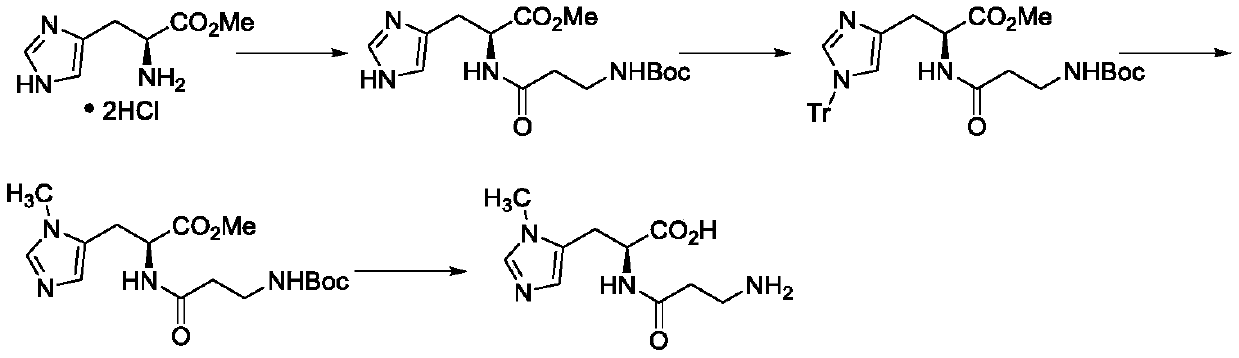
Preparation methods of two kinds of anserine and immediate of anserine
A technology of anserine and intermediates, applied in the field of medicinal chemistry, can solve the problems of unindustrialization of column chromatography, difficulty in removing protective groups, easy racemization of chiral centers, etc., and achieve convenient post-reaction treatment, easy control, and simple operation easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] The preparation of step a, compound (IV)
[0048] N(τ)-methyl-L-histidine methyl ester (10 g, 55 mmol) and 3-(tert-butoxycarbonyl)-4,5-dihydro-1,3-oxazine-2,6-dione (14.2g, 66mmol) was dissolved in 100mL acetonitrile, and triethylamine (6.6g, 65mmol) was added in batches at 20-30°C; after the addition was complete, it was stirred at 20-30°C for 4 hours; TLC detected that the reaction was complete. Concentrate, liquidize with dichloromethane water, concentrate the organic phase and recrystallize with ethyl acetate / n-heptane. After filtering and drying, 14.6 g of white solid N(α)-(3-((tert-butoxycarbonyl)amino)propylcarbonyl)-N(τ)-methyl-L-histidine methyl ester was obtained. Molar yield 75%.
[0049] Preparation of step b and step d, compound (II) and compound (I)
[0050] N(α)-(3-((tert-butoxycarbonyl)amino)propylcarbonyl)-N(τ)-methyl-L-histidine methyl ester (10 g, 28 mmol) was dissolved in 50 mL of methanol and 20 mL of water. Add 1M sodium hydroxide (...
Embodiment 2
[0057]
[0058] The preparation of step a, compound (IV)
[0059] N(τ)-methyl-L-histidine benzyl ester (10 g, 39 mmol) and 3-(tert-butoxycarbonyl)-4,5-dihydro-1,3-oxazine-2,6-dione (9.2g, 43mmol) was dissolved in 100mL tetrahydrofuran, and sodium carbonate (5.0g, 47mmol) was added in batches at 20-30°C; after the addition was complete, it was stirred at 20-30°C for 6 hours; TLC detected that the reaction was complete. Concentrate, liquidize with dichloromethane water, concentrate the organic phase and recrystallize with ethyl acetate / n-heptane. After filtering and drying, 11.4 g of white solid N(α)-(3-((tert-butoxycarbonyl)amino)propylcarbonyl)-N(τ)-methyl-L-histidine methyl ester was obtained. The molar yield is 68%.
[0060] Preparation of step b and step d, compound (II) and compound (I)
[0061] N(α)-(3-((tert-butoxycarbonyl)amino)propylcarbonyl)-N(τ)-methyl-L-histidine benzyl ester (10 g, 23 mmol) was dissolved in 50 mL of methanol and 30 mL of water. Add 10% Pd-C ...
Embodiment 3
[0068]
[0069] The preparation of step a, compound (IV)
[0070] N(τ)-methyl-L-histidine isopropyl ester (55mmol) and 3-(tert-butoxycarbonyl)-4,5-dihydro-1,3-oxazine-2,6-dione ( 44mmol) was dissolved in 100mL toluene, and 0.8 potassium bicarbonate (44mmol) was added in batches at 20-30°C; after the addition was complete, it was stirred at 20-30°C for 8 hours; TLC detected that the reaction was complete. Concentrate, liquidize with dichloromethane, and recrystallize the organic phase with ethyl acetate / n-heptane after concentration. After filtration, dry to obtain white solid N(α)-(3-((tert-butoxycarbonyl)amino)propylcarbonyl)-N(τ)-methyl-L-histidine isopropyl ester, molar yield 78% .
[0071] Preparation of step b and step d, compound (II) and compound (I)
[0072] N(α)-(3-((tert-butoxycarbonyl)amino)propylcarbonyl)-N(τ)-methyl-L-histidine isopropyl ester (28mmol) was dissolved in 50mL methanol and 20mL water, 20- Add 1M formic acid (28mL) at 30°C, stir at this tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com