Core-shell CO oxidation catalyst as well as preparation method and application thereof
An oxidation catalyst, core-shell type technology, applied in the direction of catalyst activation/preparation, physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, etc., can solve the complex preparation process, high production cost, Less storage capacity and other issues, to achieve the effects of avoiding catalyst deactivation, prolonging service life, and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
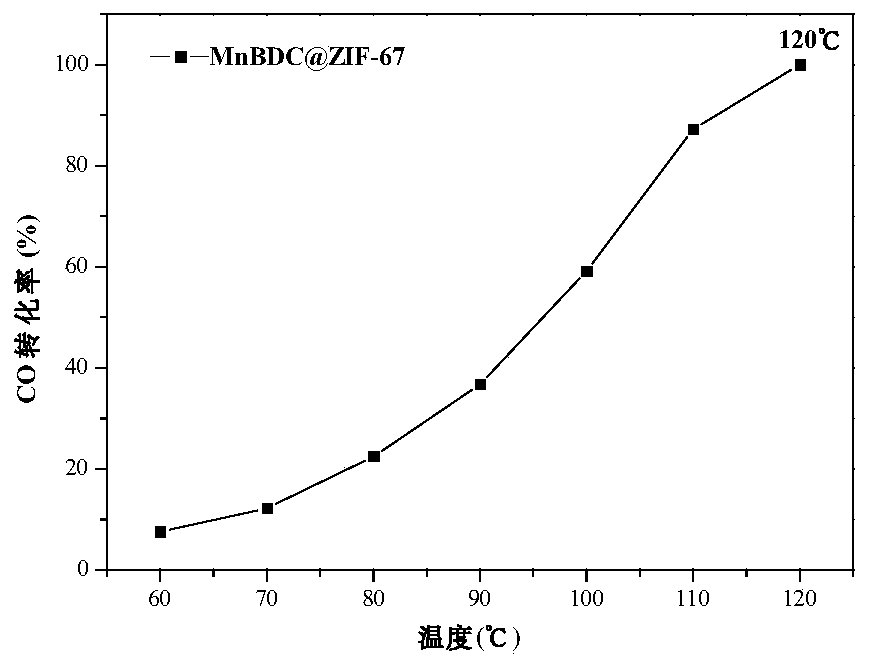
[0039] Example 1: Preparation of core-shell catalyst derived from MnBDC@ZiF-67
[0040] First, dissolve 0.6125g of manganese acetate tetrahydrate and 0.4150g of terephthalic acid in 25mL of N,N-dimethylformamide, mix them uniformly by ultrasonic, and record it as solution A; then mix 1.4550g of cobalt nitrate hexahydrate with 1.6460g 2-Methylimidazole was dissolved in 40 mL of methanol respectively, and mixed evenly after complete dissolution, which was recorded as solution B; the mixed solutions A and B were ultrasonically stirred, and placed in an autoclave at 150 °C for 5 h. After centrifugal washing with DMF for 2-3 times, washing with methanol until the supernatant was colorless, and drying at 80°C, the MnBDC@ZiF-67 core-shell material was obtained, which was purple in color. Then calcined at 350° C. for 2 h in a muffle furnace to obtain the final catalyst, which was black in color.
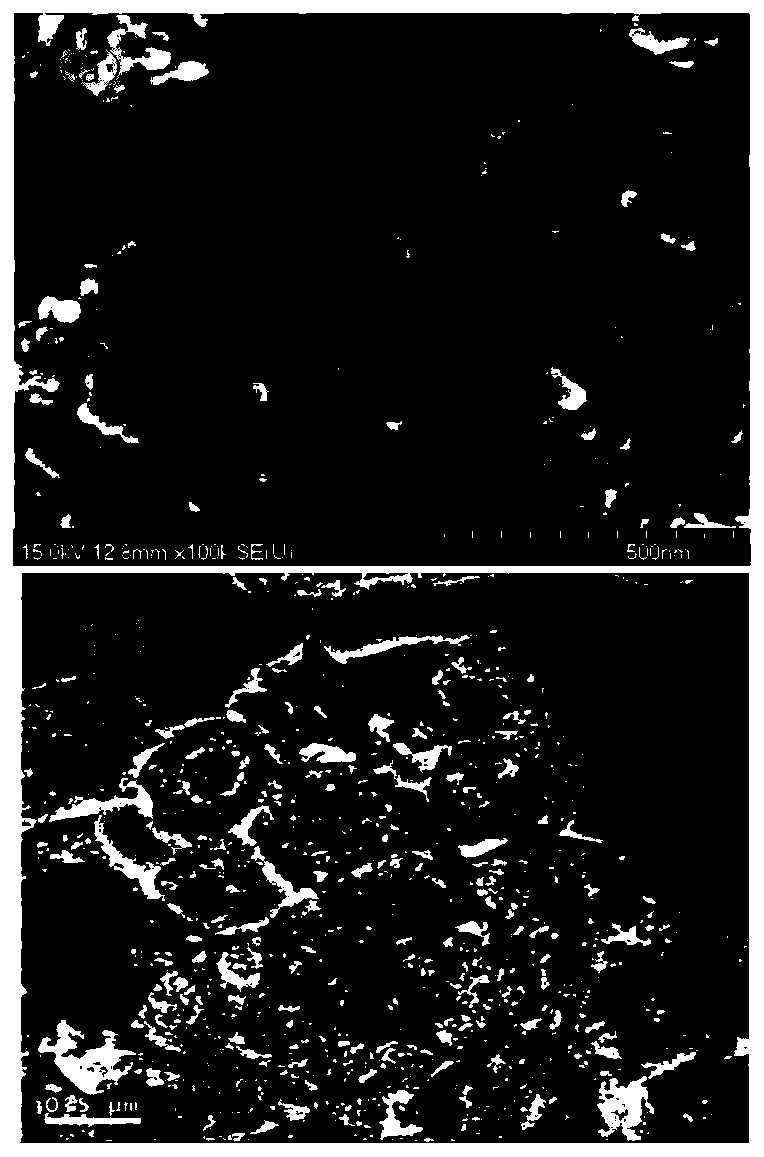
[0041] The CO catalytic test analysis was carried out on the core-shell catalyst derive...
Embodiment 2
[0044] Example 2: Preparation of core-shell catalyst derived from FeBTC (hydrothermal)@ZiF-67
[0045] First, dissolve 0.4000g of FeBTC (hydrothermal) in 40mL of ethanol, mix it uniformly by ultrasonic, and record it as solution A; then dissolve 1.4550g of cobalt nitrate hexahydrate and 1.6420g of 2-methylimidazole in 40mL of methanol, and completely dissolve After mixing evenly, record it as solution B; mix A and B solutions, and stir at room temperature for 24 hours. After centrifugation and washing with ethanol, the supernatant was washed with methanol until it was colorless, and dried at 80° C. to obtain an uncalcined product with a purple color. Then calcined at 350° C. for 2 h in a muffle furnace to obtain the final catalyst, which was black in color.
[0046] The CO catalytic test analysis was carried out on the core-shell catalyst derived from FeBTC (hydrothermal)@ZiF-67, and CO was completely oxidized at 110 °C.
Embodiment 3
[0047] Example 3: Preparation of core-shell catalyst derived from CuBTC (nitrate, 100°C, hydrothermal)@ZiF-67
[0048] First, dissolve 0.4000g CuBTC (nitrate, 100°C, hydrothermal) in 40mL of ethanol, mix it uniformly by ultrasonic, and record it as solution A; then dissolve 1.4550g of cobalt nitrate hexahydrate and 1.6420g of 2-methylimidazole in 40mL In methanol, completely dissolved and mixed evenly, recorded as solution B; mix A and B solutions, and stir at room temperature for 24 hours. After centrifugation and washing with ethanol, the supernatant was washed with methanol until it was colorless, and dried at 80° C. to obtain an uncalcined product with a purple color. Then it was calcined at 350°C for 2 h in a muffle furnace to obtain the final catalyst, which was black in color.
[0049] The CO catalytic test analysis was carried out on the core-shell catalyst derived from CuBTC (nitrate, 100 °C, hydrothermal) @ZiF-67, and CO was completely oxidized at 110 °C. Scanning ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com