Material with long-service-life room-temperature phosphorescence phenomenon, preparation method and application
A room-temperature phosphorescence and long-life technology, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve problems such as the difficulty in synthesis of inorganic long-lasting materials and the doping of heavy metal elements, and achieve cheap raw materials, simple preparation, and wide application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Synthesis of compound Ben-H.
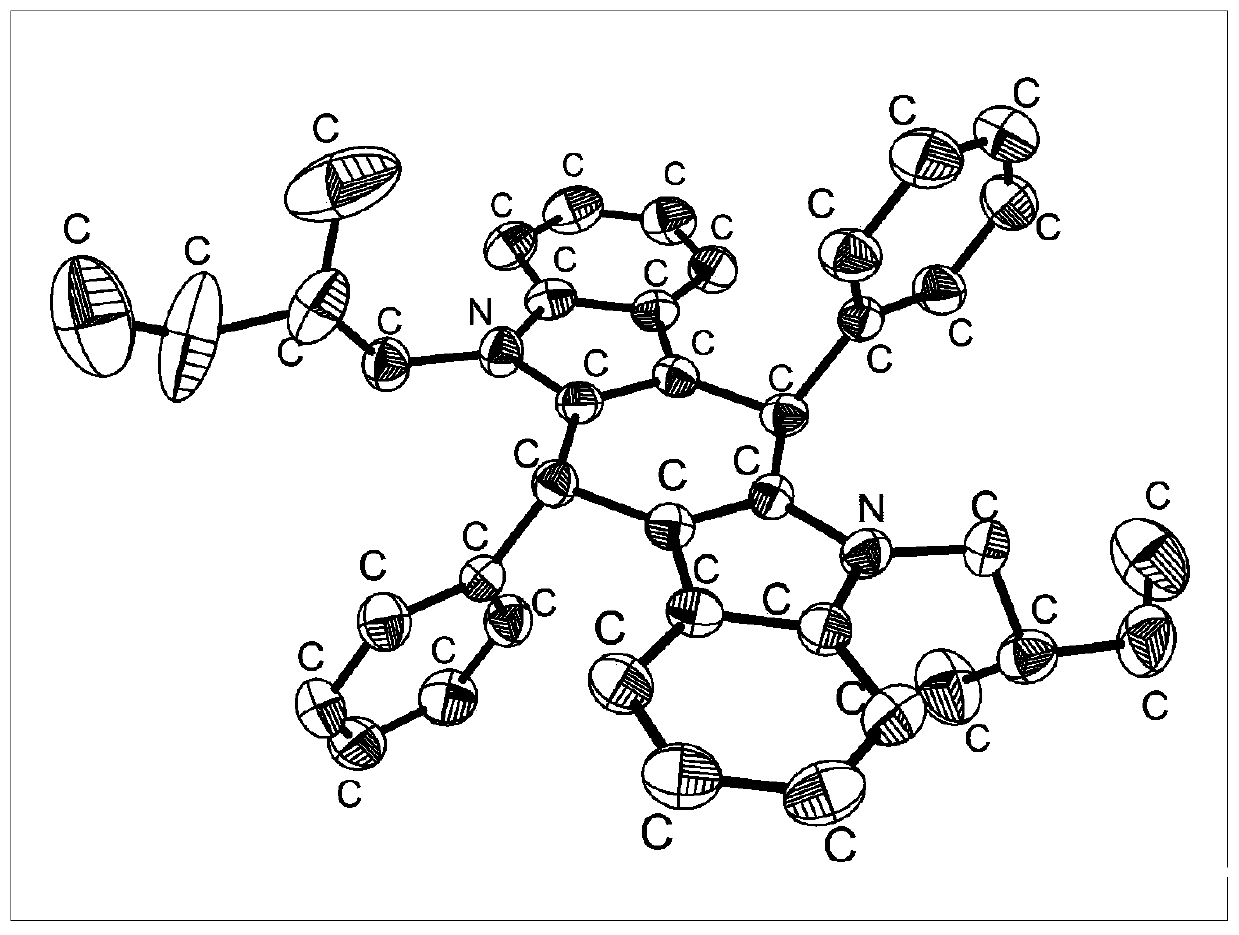
[0030] Dissolve 1.17g of indole and 1.06g of benzaldehyde in anaerobic dichloromethane, add 0.1mL trifluoroacetic acid in the dark, react in the dark for 24 hours at room temperature, filter the solid with suction and wash with dichloromethane to obtain Compound Ben-H, the yield is 36.5%. 1 H NMR (400MHz, DMSO-d 6 )δ=10.70(s, 2H), 7.34(d, J=7.5Hz, 4H), 7.29(t, J=7.4Hz, 4H), 7.22(dd, J=14.9, 6.9Hz, 4H), 7.08( d, J=7.9Hz, 2H), 6.95(t, J=7.6Hz, 2H), 6.79(t, J=7.5Hz, 2H), 5.70(s, 2H). The compound is dissolved in dimethyl sulfoxide, and crystals are obtained after standing still for a period of time, and the thermal ellipsoid diagram of the crystal is shown in figure 1 shown. The synthetic route is as follows:
[0031]
Embodiment 2
[0032] Example 2: Synthesis of compound Ben-Ox.
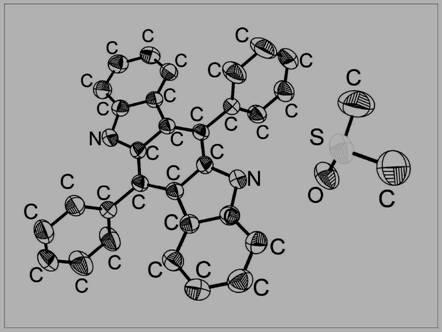
[0033] 410.5 mg of Ben-H and 507.6 mg of iodine were dissolved in 30 mL of acetonitrile solvent and reacted in the dark for 8 hours under heating at 80 degrees Celsius. The solid was obtained by suction filtration and washed with cold acetonitrile to obtain the compound Ben-Ox with a yield of 44.1%. 1 H NMR (400MHz, DMSO-d 6 )δ=10.53(s, 2H), 7.78-7.63(m, 10H), 7.45(d, J=8.0Hz, 2H), 7.29-7.22(m, 2H), 7.06(d, J=8.1Hz, 2H ), 6.87-6.77 (m, 2H). The compound is dissolved in dimethyl sulfoxide, and crystals are obtained after standing still for a period of time, and the thermal ellipsoid diagram of the crystal is shown in figure 2 shown. The synthetic route is as follows:
[0034]
Embodiment 3
[0035] Example 3: Synthesis of Compound Ben-2S.
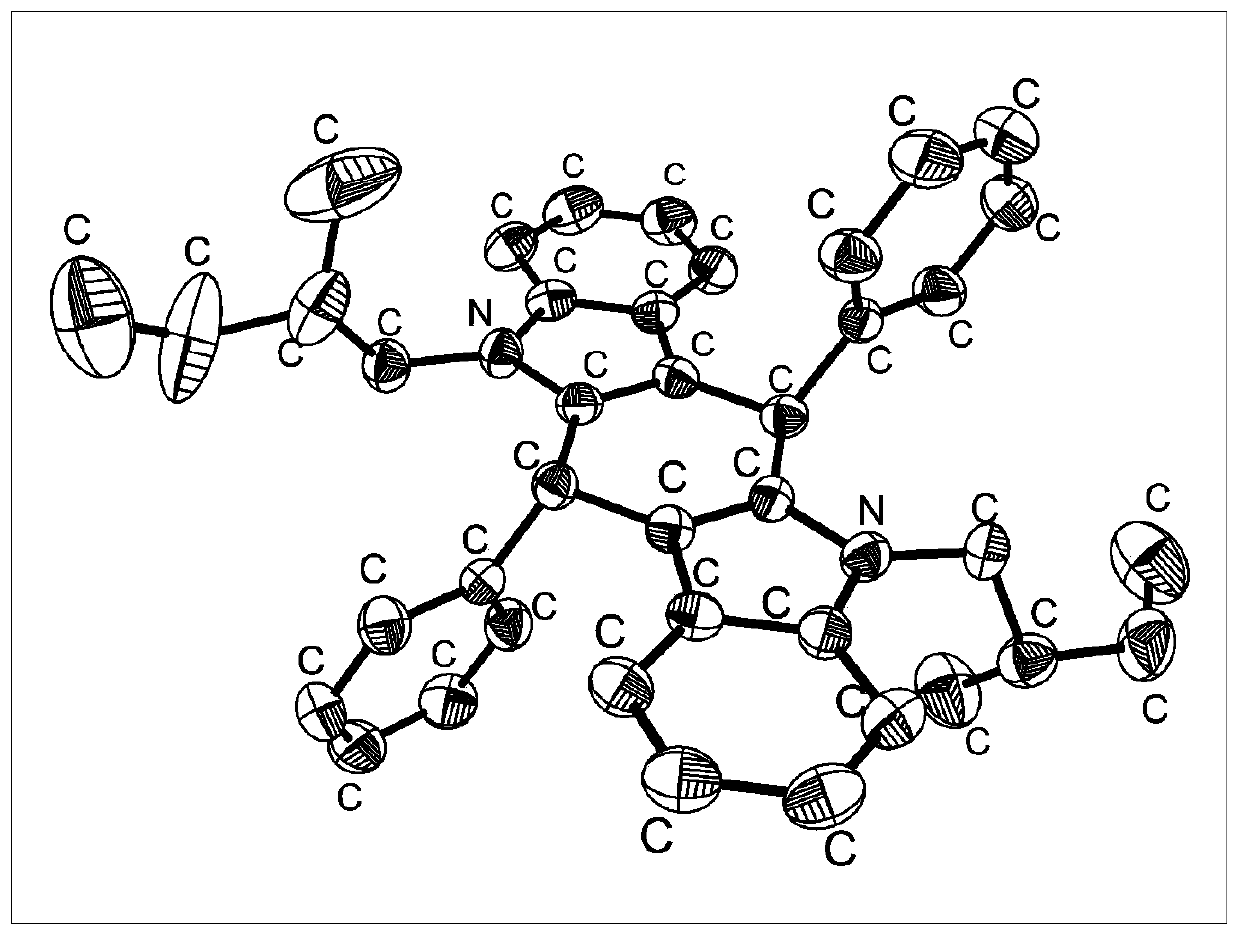
[0036] Dissolve 410.5mg of Ben-H and 5mg of benzyltriethylammonium chloride in 20mL of dimethyl sulfoxide, add 1mL of potassium hydroxide aqueous solution (50% mass ratio) under nitrogen atmosphere, and then slowly add 377.6mg of (S) -1-bromo-2-methylbutane, reacted at room temperature for 2 hours, added a large amount of water to wash away dimethyl sulfoxide to obtain a solid, separated by column chromatography, and the eluent ratio was dichloromethane / petroleum ether= 1:5, the compound Ben-2S was obtained with a yield of 51%. 1 H NMR (400MHz, CDCl 3 )δ=7.52(t, J=7.1Hz, 2H), 7.34(d, J=7.3Hz, 4H), 7.28-7.22(m, 4H), 7.22-7.13(m, 4H), 7.10(m, 2H ), 6.98(m, 2H), 5.82(q, J=3.5Hz, 2H), 3.87-3.58(m, 4H), 1.96-1.80(m, 2H), 1.43-1.28(m, 2H), 1.26- 1.10 (m, 2H), 0.94-0.84 (m, 9H), 0.74 (d, J=6.6Hz, 3H). The compound is dissolved in dichloromethane, and crystals are obtained after standing still for a period of time, and the thermal ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com