Gene drug constructs for the treatment of mucopolysaccharidosis type 3a
A technology of gene medicine and gene expression, applied in drug combination, gene therapy, genetic engineering, etc., can solve the problem of low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Construction of plasmid vector
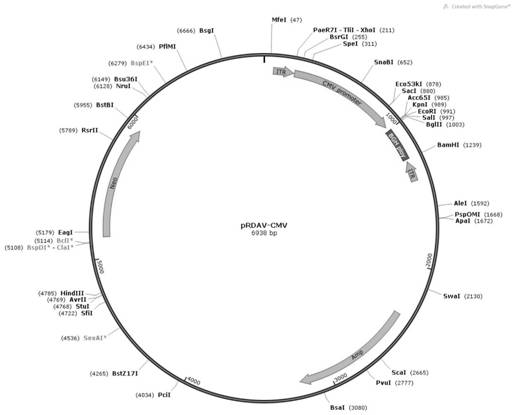
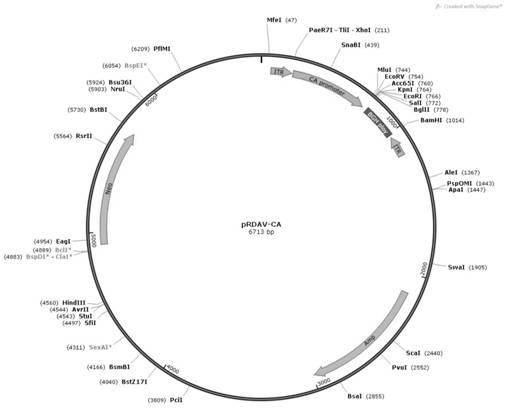
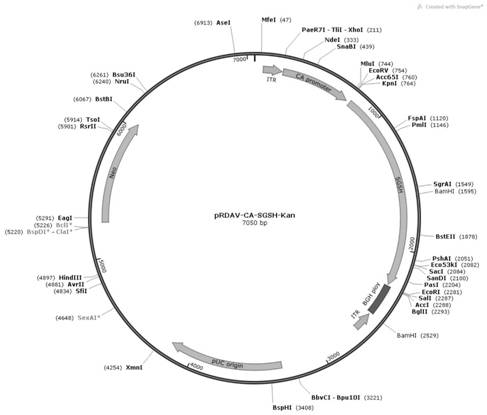
[0051] In order to construct the pRDAV-CA-SGSH plasmid required for packaging recombinant AAV virus, we first used the pRDAV-CMV ( figure 1 ) as the basis, the CMV promoter in the pRDAV vector was replaced with the self-designed CA promoter (SEQ ID No.1) to obtain the pRDAV-CA vector. Next, clone the artificially synthesized human SGSH (SEQ ID No.2) sequence into the pRDAV-CA vector Kpn I and EcoR Between the I restriction sites, pRDAV-CA-SGSH and the vector were obtained.
[0052] (1) Construction of pRDAV-CA vector
[0053] The human cytomegalovirus early gene enhancer sequence and the chicken β-actin promoter sequence were spliced to obtain the CA promoter sequence, and the sequence information is shown in SEQ ID No.1. Add at both ends of the CA promoter sequence xho I and Kpn I restriction site. After adding restriction sites, the sequence was synthesized by GenScript Biotechnology Co., Ltd., and the synthetic...
Embodiment 2
[0062] Example 2 In vitro expression verification of pRDAV-CA-SGSH vector
[0063] (1) Expression determination of SGSH protein
[0064] The well-grown Huh-7 cells were evenly spread in 9 wells of a six-well cell culture plate, and when the cell density in each well reached 80%, they were transfected with pRDAV-CA-SGSH and 3 wells for each pscAAV-CMR-SGSH (refer to the instructions for the detailed process). After 48 h of transfection, the cells were collected after digestion, and the total protein of the cells was extracted by repeated freezing and thawing followed by centrifugation. The total protein concentrations of transfected pRDAV-CA-SGSH, pscAAV-CMR-SGSH and blank cells were measured using Pierce BCA Protein Aaasy Kit (ThermoFisher, USA), and the detailed process refers to the kit instructions.
[0065] After extracting the total protein of Huh-7 cells, Western Blotting was used to detect the expression of SGSH protein in the cells. For the method, see "Molecular Clo...
Embodiment 3
[0070] Example 3 Preparation and assay of rAAV-SGSH
[0071] (1) Packaging of different serotype recombinant AAV viruses
[0072] Referring to literature [64], the three-plasmid packaging system was used to package and purify the recombinant AAV virus. Briefly, the AAV vector plasmid (pRDAV-CA-SGSH or pscAAV-CMR-SGSH), the helper plasmid (pHelper) and the AAV Rep and Cap protein expression plasmids (pAAV-R2C5, pAAV-R2C9 or pAAV-R2C10) were prepared according to 1: After mixing at a molar ratio of 1:1, HEK293 cells were transfected by the calcium phosphate method. After 48 hours of transfection, the cells and culture supernatant were harvested, and the recombinant AAV virus was isolated and purified by cesium chloride density gradient centrifugation. Packaged and purified to obtain rAAV5-CA-SGSH, rAAV9-CA-SGSH, rAAVrh10-CA-SGSH, rscAAV5-CMR-SGSH, rscAAV9-CMR-SGSH, rscAAVrh10-CMR-SGSH.
[0073] (2) Packaging of rAAVDJ-SGSH
[0074] rAAVDJ-CA-SGSH and rAAVDJ-CMR-SGSH were pack...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com