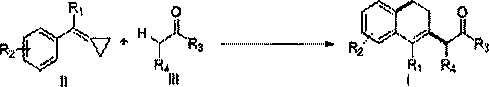
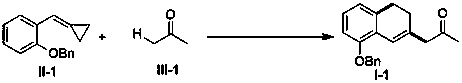Synthesis method of 3,4-dihydronaphthalene compound substituted by alkoxy
A synthesis method and compound technology, which can be used in the preparation of carbon-based compounds, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of high cost, harsh reaction conditions, and low adaptability of reaction substrates.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
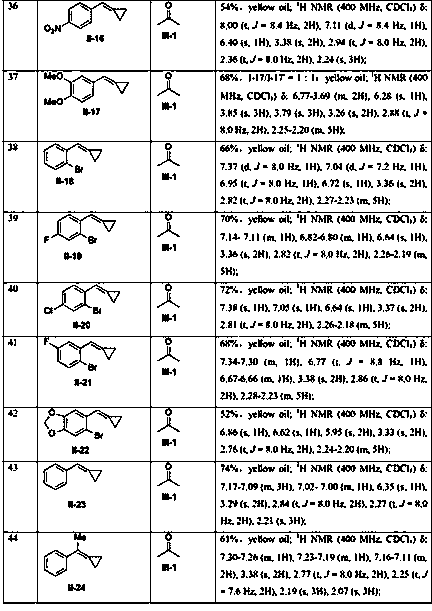
Examples
Embodiment 1
[0051] Add methylenyl cyclopropane compound (0.2 mmol) shown in formula II-1 and oxone (chemical formula is KHSO 5 , 0.4 mmol, 2 equiv), followed by the addition of acetone (2 mL). Replace the air in the reactor with argon for 3-5 times, heat and react in an argon atmosphere at 90°C in an oil bath for 24 hours, check that the raw materials have reacted completely by TLC and / or GC-MS, and then filter the reaction solution , the organic phase was evaporated to dryness with a rotary evaporator, and the residue was separated and purified by column chromatography (eluent: petroleum ether / ethyl acetate, volume ratio 10:1) to obtain the target product shown in formula I-1. Yield 61%. yellow oily liquid; 1 H NMR (400 MHz, CDCl 3 ) δ: 7.45-7.37 (m, 4H), 7.35-7.31 (m, 1H), 7.06(t, J = 8.0 Hz, 1H), 6.81 (s, 1H), 6.76 (t, J = 7.6 Hz, 2H), 5.07 (s, 2H), 3.30 (s, 2H), 2.81 (t, J = 8.0 Hz, 2H), 2.24 (t, J = 8.0 Hz, 2H), 2.19 (s,3H); 13 C { 1 H}NMR (100 MHz, CDCl 3 ) δ: 207.0, 15...
Embodiment 2
[0053] Add the methylenecyclopropane compound (0.2 mmol), AgOTf (0.02 mmol, 10 mol %) and oxone (chemical formula KHSO 5 , 0.4 mmol, 2 equiv), followed by the addition of acetone (2 mL). Replace the air in the reactor with argon for 3-5 times, heat and react in an argon atmosphere at 90°C in an oil bath for 24 hours, check that the raw materials have reacted completely by TLC and / or GC-MS, and then filter the reaction solution , the organic phase was evaporated to dryness with a rotary evaporator, and the residue was separated and purified by column chromatography (eluent: petroleum ether / ethyl acetate, volume ratio 10:1) to obtain the target product shown in formula I-1. Yield 68%.
Embodiment 3
[0055] Consistent with Example 2, the only difference is that AgOAc is used instead of AgOTf. Yield 63%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com