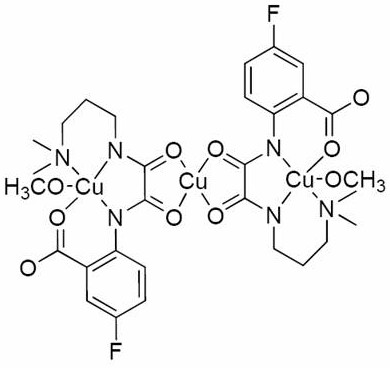
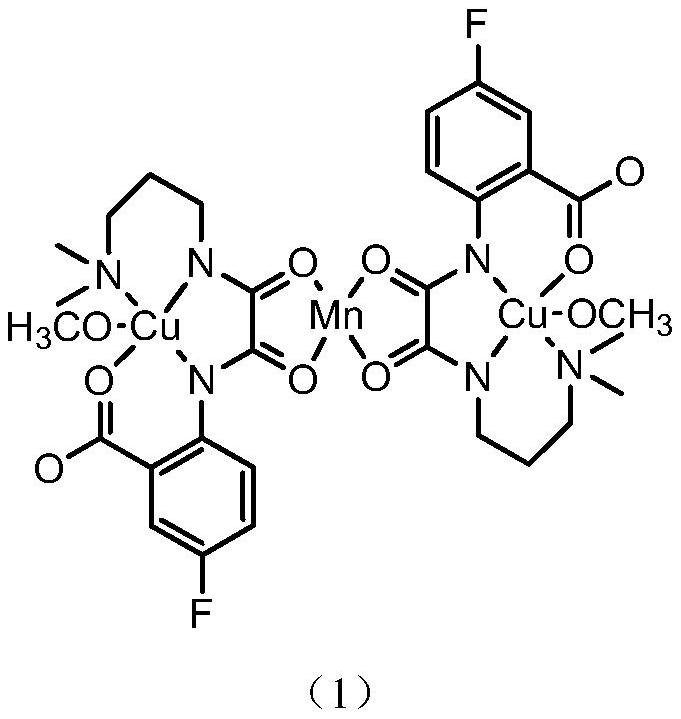
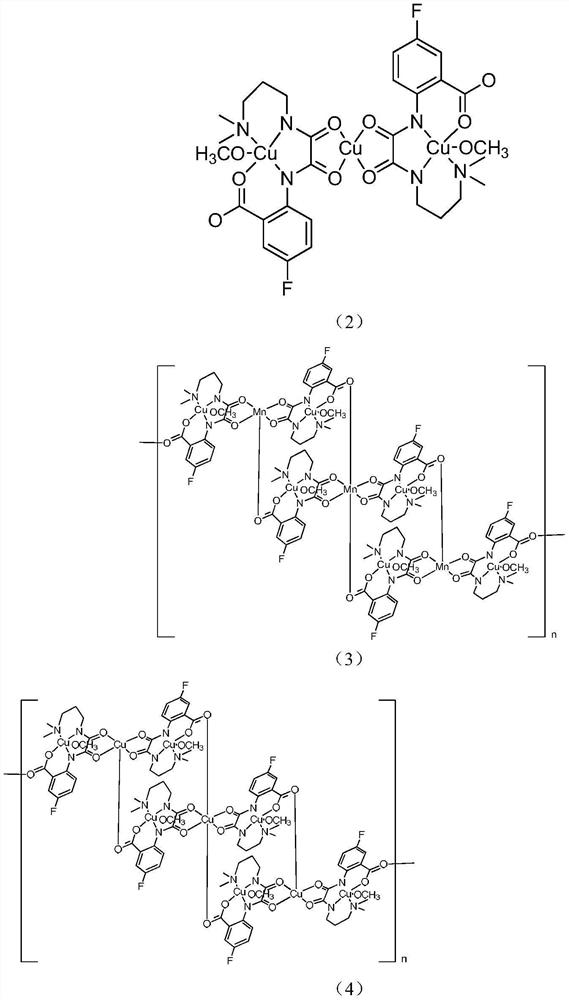
Preparation method and application of a kind of fluorine-containing oxamide homo/heteronuclear compound
A technology of oxamide and metal compounds, applied in the application field of insecticidal active drugs, can solve the problems of unreported insecticidal activity research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Step 1: Add 10.0mmol of 2-amino-5-fluorobenzoic acid to the reaction vessel, dissolve it with 20ml of tetrahydrofuran, dilute 12.0mmol of ethyl oxalyl chloride with 8ml of tetrahydrofuran and add it dropwise to the above solution, in an ice-water bath Reacted for 1 hour, evaporated by rotary evaporation, washed with cold ethanol, and dried under vacuum to obtain a white powdery solid. Yield: 85%.
[0020] 1H-NMR(400MHz,DMSO)δ14.28(s,1H),12.47(s,1H),8.64-8.60(dd,J=5.2Hz,1H),7.79-7.76(m,1H),7.61-7.56 (m,1H),4.35-4.29(q,2H),1.35-1.31(t,3H)
[0021] Step 2: Dissolve 10.0 mmol of the white powder obtained in the previous step reaction in 30 ml of tetrahydrofuran, dilute 15.0 mmol of N,N-dimethyl-1,3-propanediamine with 20 ml of tetrahydrofuran in an ice-water bath, and then drop by drop The above solution was added, stirred overnight at room temperature, evaporated on a rotary basis, washed successively with cold tetrahydrofuran and cold ethanol, and dried in vacuo to obta...
Embodiment 2
[0047] Step 1: Add 10.0mmol of 2-amino-5-fluorobenzoic acid to the reaction vessel, dissolve it with 20ml of tetrahydrofuran, dilute 12.0mmol of ethyl oxalyl chloride with 8ml of tetrahydrofuran and add it dropwise to the above solution, in an ice-water bath Reacted for 1 hour, evaporated by rotary evaporation, washed with cold ethanol, and dried under vacuum to obtain a white powdery solid. Yield: 85%. 1H NMR (400MHz, DMSO) δ14.28(s, 1H), 12.47(s, 1H), 8.64-8.60(dd, J=5.2Hz, 1H), 7.79-7.76(m, 1H), 7.61-7.56( m,1H),4.35-4.29(q,2H),1.35-1.31(t,3H)
[0048] Step 2: Dissolve 10.0 mmol of the white powder obtained in the previous step reaction in 30 ml of tetrahydrofuran, dilute 15.0 mmol of N,N-dimethyl-1,3-propanediamine with 20 ml of tetrahydrofuran in an ice-water bath, and then drop by drop The above solution was added, stirred overnight at room temperature, evaporated on a rotary basis, washed successively with cold tetrahydrofuran and cold ethanol, and dried in vacuo to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com