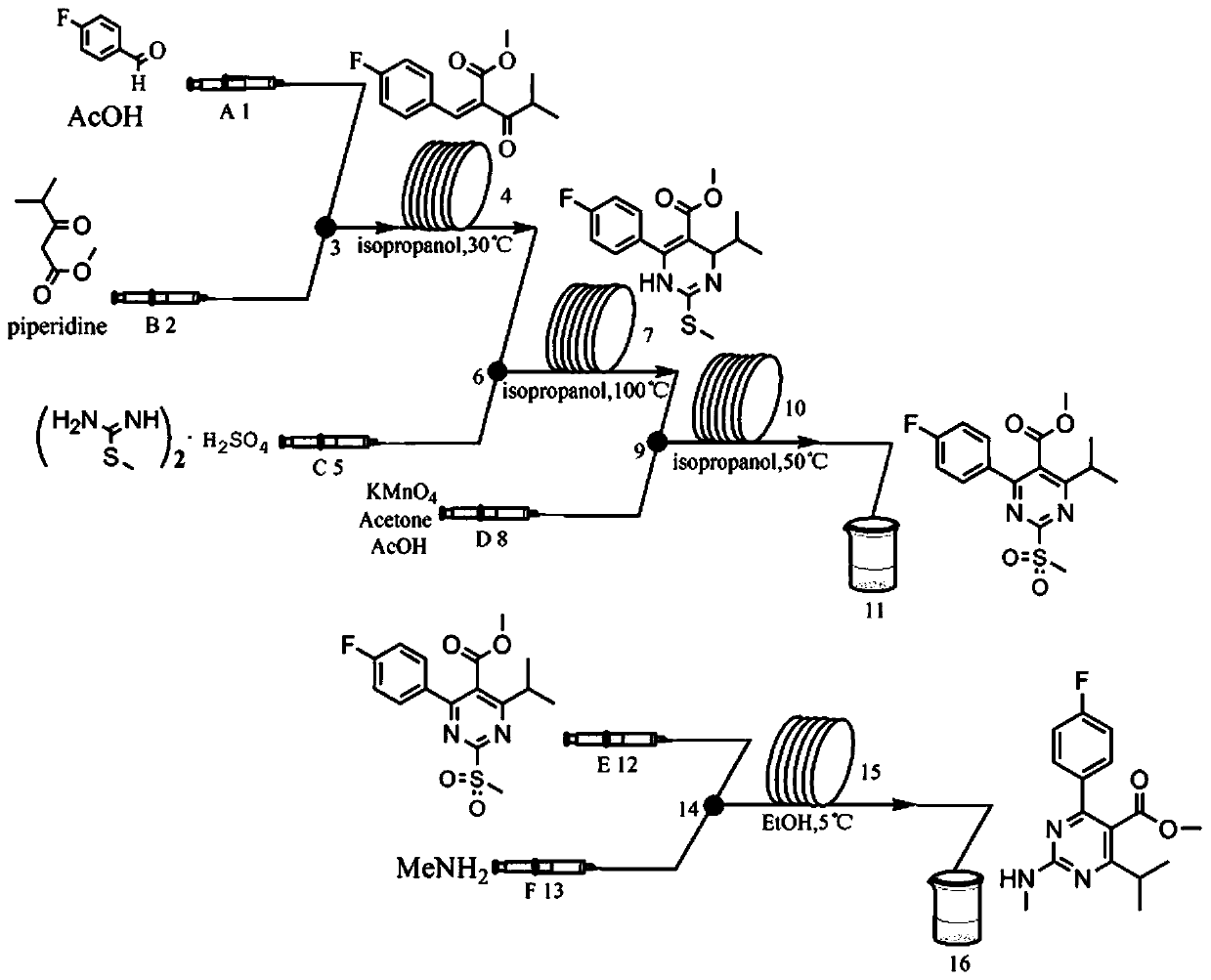
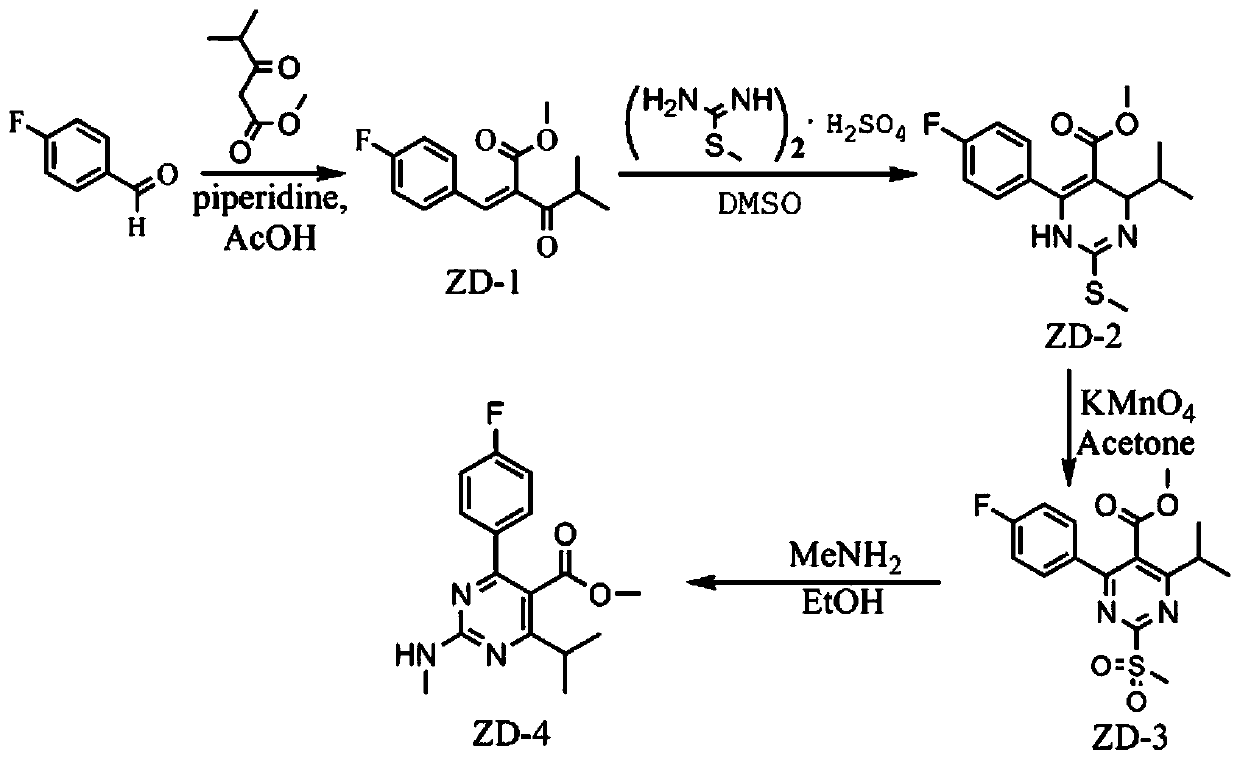
Method for continuously preparing rosuvastatin intermediate by micro-channel modular reaction device
A microchannel module, rosuvastatin technology, applied in chemical recovery, organic chemistry and other directions, can solve the problems of low yield, difficult to purify, many by-products, etc., and achieve high yield, easy operation, and less side reactions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
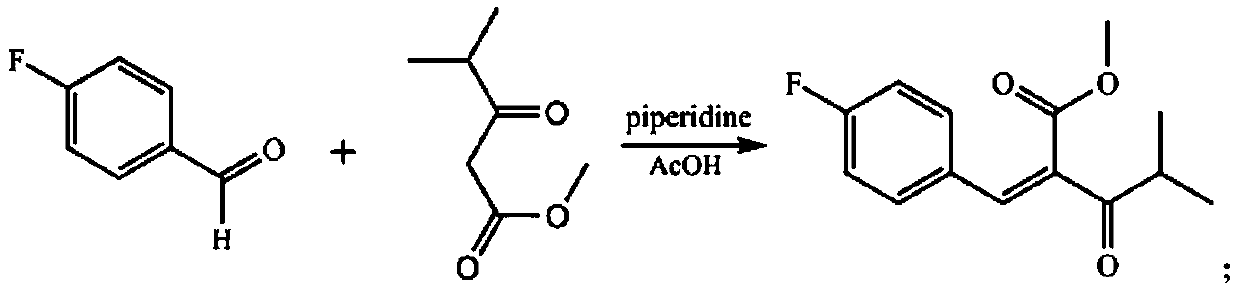
[0046] (1) In the microchannel reaction device, the isopropanol solution (11.5mL) of p-fluorobenzaldehyde (0.04mol) and the isopropanol solution (0.135mL) of acetic acid catalyst (0.135mL), methyl isobutyrylacetate (0.04mol) Solution (11.5mL) and catalytic amount of piperidine (0.225mL) were pumped into the first mixer 3 from pump A1 and pump B2 respectively, the flow rate of pump A1 was 0.38mL / min, and the flow rate of pump B2 was 0.38mL / min min. After fully mixing, enter the first microreactor 4. The volume of the first microreactor 4 is 40mL, the reaction residence time is 30min, and the reaction temperature is 30°C. The obtained reaction effluent is 2-(4-fluorophenyl)methylene - Methyl 3-oxo-4-methylpentanoate (ZD-1).
[0047] (2) along with the reaction solution that step (1) obtains flows into the second mixer 6, flow velocity is 1.24mL / min, S-methylisothiouronium sulfate (2.08mol) is added into equimolar amount of saturated sodium bicarbonate ( 2.08mol) free isopropan...
Embodiment 2
[0060] The operation is the same as in Example 1, the only difference is:
[0061] In step (1), the reaction temperature in the first microreactor is 20°C, and finally 2-(4-fluorophenyl)methylene-3-oxo-4-methylpentanoic acid methyl ester (ZD- 1) The reaction effluent has a yield of 75%.
Embodiment 3
[0063] The operation is the same as in Example 1, the only difference is:
[0064] In step (1), the reaction temperature in the first microreactor is 40°C, and finally 2-(4-fluorophenyl)methylene-3-oxo-4-methylpentanoic acid methyl ester (ZD- 1) The reaction effluent has a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com