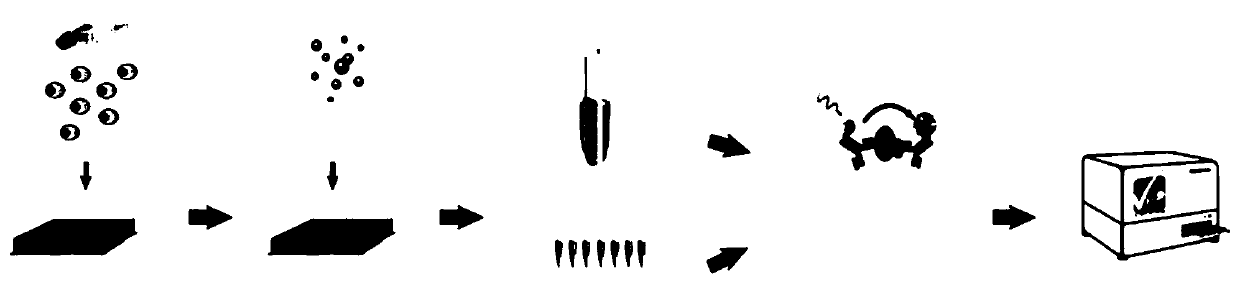
Method of detecting multiple cell factors simultaneously by HTFR (homogeneous time-resolved fluorescence) technique
A technology of cytokines and cells, applied in biological testing, material inspection products, fluorescence/phosphorescence, etc., can solve problems such as expensive, difficult to popularize, and rising detection costs, and achieve low false negative rate, stable signal, and low cost. controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
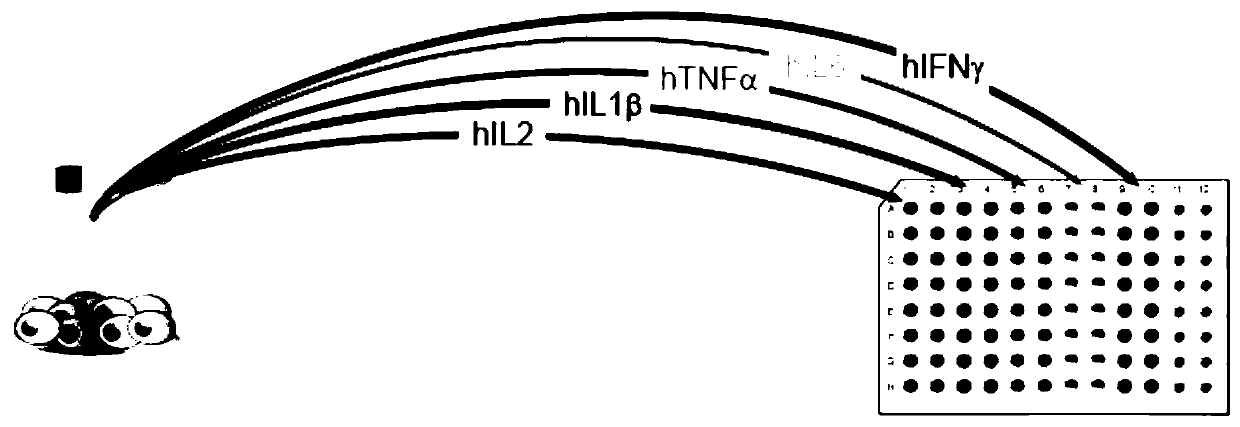
[0047] like Figure 4 as shown, Figure 4 Shown is the release of cytokines by dexamethasone under the combined action of propylene glycol methyl ether acetate (PMA) and ionomycin.
[0048] Figure 4 Interleukin 2 (IL2), Interleukin 6 (IL6), Tumor Scurvy Factor α (TNFα) and Interferon detected by HTRF in the same PBMC sample stimulated with propylene glycol methyl ether acetate (PMA) and ionomycin γ(IFNγ), from Figure 4 As seen in the left panel, this stimulation significantly increased the release of all cytokines, as expected.
[0049] In this example, propylene glycol methyl ether acetate is a small molecular organic compound that can directly penetrate the cell membrane and activate PKC enzymes without the need for activation of receptors on cells. Ionomycin is a calcium ionophore that activates calcium ion release, thereby activating downstream signaling pathways.
[0050] These two compounds act together to fully activate cytokine release from all cells in PBMCs, wit...
Embodiment 2
[0054] like Figure 5 As shown, under the action of dexamethasone, cytokines in B cells were released.
[0055] In this example, LPS is a specific stimulating factor for B cells in PBMCs. The experimental results obtained by using HTRF are as expected. After it stimulates B cells, the secretion of IL1beta, IL6 and TNFalpha cytokines is significantly increased. After co-stimulation with dexamethasone, more than 60% of the stimulating effect of LPS can be effectively reversed.
Embodiment 3
[0057] like Image 6 As shown, under the action of dexamethasone, cytokines in T cells were released.
[0058] In this example, CD3 and CD28 antibodies are widely used to stimulate T cells in PBMC, and after stimulating T cells, the secretion of IL2 and IFNgamma is significantly increased. The experimental results obtained with HTRF are as expected. After co-stimulation with dexamethasone, more than 90% of the stimulating effect of CD3 / CD28 can be effectively reversed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com