a w 18 o 49 /niwo 4 Preparation method of /nf self-supporting electrocatalytic material
An electrocatalytic material and self-supporting technology, applied in chemical instruments and methods, physical/chemical process catalysts, electrodes, etc., can solve problems such as low specific surface area, poor conductivity, instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1) Take Na at a molar ratio of 1:1 2 WO 4 2H 2 O and Ni(CH 3 COO)2 4H 2 O, the Na 2 WO 4 2H 2 O and Ni(CH 3 COO) 2 4H 2 O was added to 40mL deionized water and stirred evenly to obtain Na 2 WO 4 2H 2 O concentration is 0.05mol / L, Ni(CH 3 COO) 2 4H 2 O concentration is the mixed solution A of 0.05mol / L;
[0034] 2) Put the mixed solution A into a polytetrafluoroethylene-lined autoclave with a volume filling ratio of 40%, seal the autoclave and put it into a homogeneous hydrothermal reactor for 12 hours at 160°C;
[0035] 3) Cool to room temperature after the reaction, centrifuge and wash the reactant three times with absolute ethanol and deionized water respectively, and dry the reactant after centrifugal washing in a vacuum oven or freeze drying oven at 50°C for 5 hours to obtain powder B;
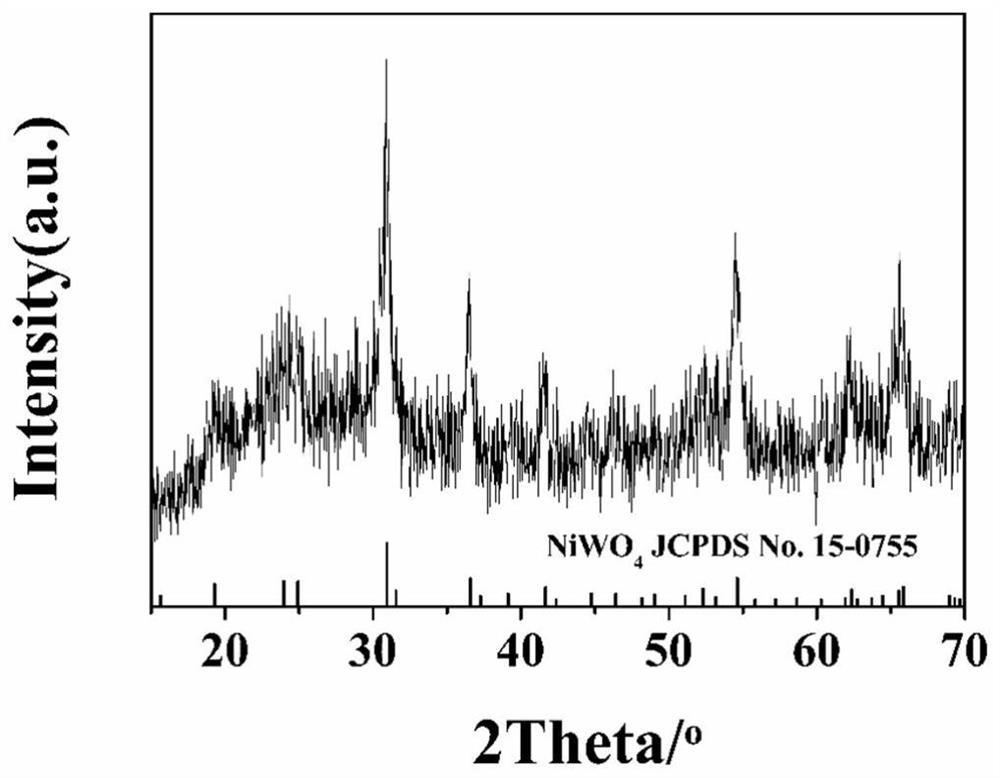
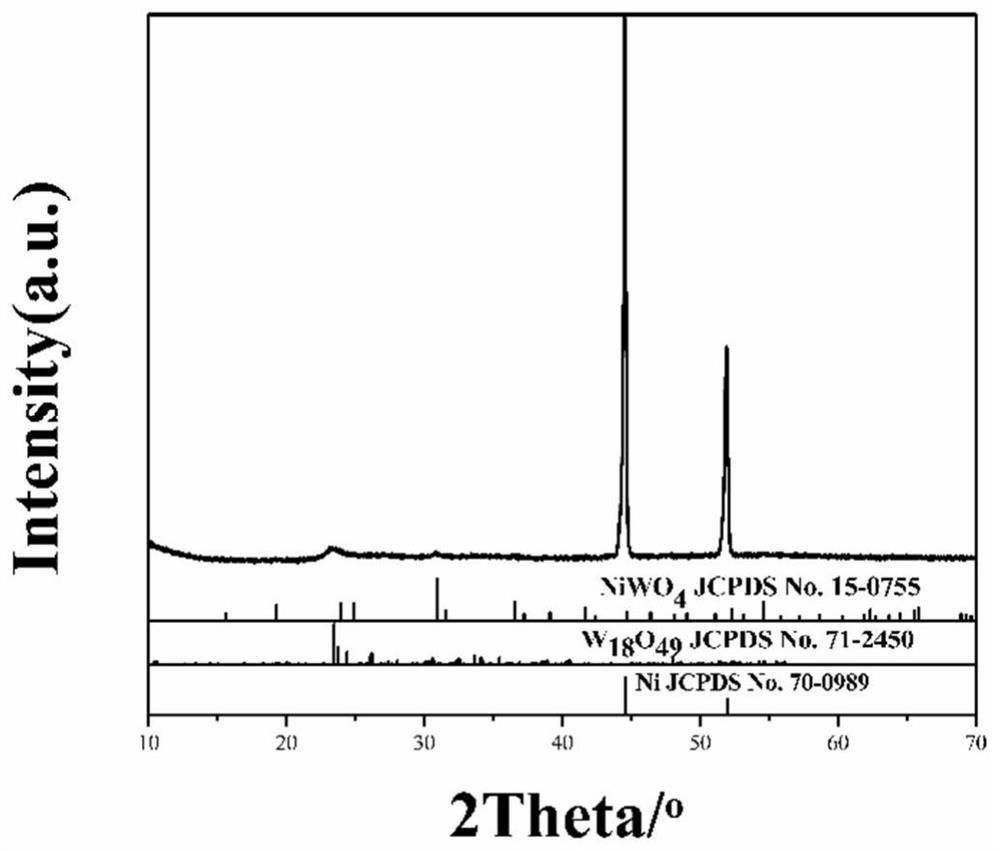
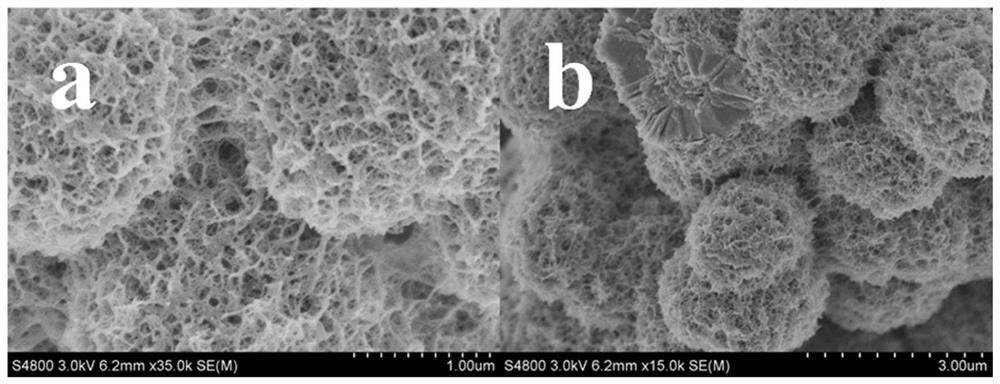
[0036] 4) Grind powder B in a mortar and put it into a muffle furnace to heat up from room temperature to 450°C at a heating rate of 2°C / min for calcination to obtai...
Embodiment 2
[0045] 1) Take Na2WO4·2H at a molar ratio of 1:1 2 O and Ni(CH 3 COO) 2 4H 2 O, the Na 2 WO4·2H 2 O and Ni(CH 3 COO) 2 4H 2 O was added to 30mL deionized water and stirred evenly to obtain Na 2 WO 4 2H 2 O concentration is 0.03mol / L, Ni(CH 3 COO) 2 4H 2 O concentration is the mixed solution A of 0.03mol / L;
[0046] 2) Put the mixed solution A into a polytetrafluoroethylene-lined autoclave with a volume filling ratio of 30%, seal the autoclave and put it into a homogeneous hydrothermal reactor for 12 hours at 180°C;
[0047] 3) Cool to room temperature after the reaction, centrifuge and wash the reactant three times with absolute ethanol and deionized water respectively, and dry the reactant after centrifugal washing in a vacuum oven or freeze drying oven at 50°C for 5 hours to obtain powder B;
[0048] 4) Grind powder B in a mortar and put it into a muffle furnace to heat up from room temperature to 470°C at a heating rate of 2°C / min for calcination to obtain NiW...
Embodiment 3
[0057] 1) Take Na at a molar ratio of 1:1 2 WO 4 2H 2 O and Ni(CH 3 COO) 2 4H 2 O, the Na 2 WO 4 2H 2 O and Ni(CH 3 COO) 2 4H 2 O was added to 35mL deionized water and stirred evenly to obtain Na 2 WO 4 2H 2 O concentration is 0.08mol / L, Ni(CH 3 COO) 2 4H 2 O concentration is the mixed solution A of 0.08mol / L;
[0058] 2) Put the mixed solution A into a polytetrafluoroethylene-lined autoclave with a volume filling ratio of 35%, seal the autoclave and put it into a homogeneous hydrothermal reactor for 28 hours at 150°C;
[0059] 3) Cool to room temperature after the reaction, centrifuge and wash the reactant three times with absolute ethanol and deionized water respectively, and dry the reactant after centrifugal washing in a vacuum oven or freeze drying oven at 50°C for 6 hours to obtain powder B;
[0060] 4) Grind powder B in a mortar and put it into a muffle furnace to heat up from room temperature to 450°C at a heating rate of 2°C / min for calcination to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com