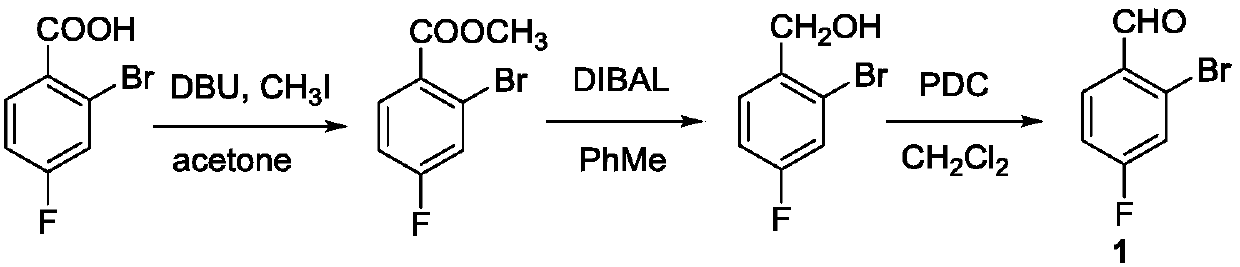
Preparation method of 2-bromine-4-fluorobenzaldehyde
A technology of fluorobenzaldehyde and bromination reagent, applied in the field of preparation of 2-bromo-4-fluorobenzaldehyde, can solve the problem that 2-bromo-4-fluorobenzonitrile is expensive, has no commercial value, and cannot be scaled up and other problems, to achieve the effect of optimizing purification steps, good industrialization potential, production and application prospects, and reducing unit quality price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
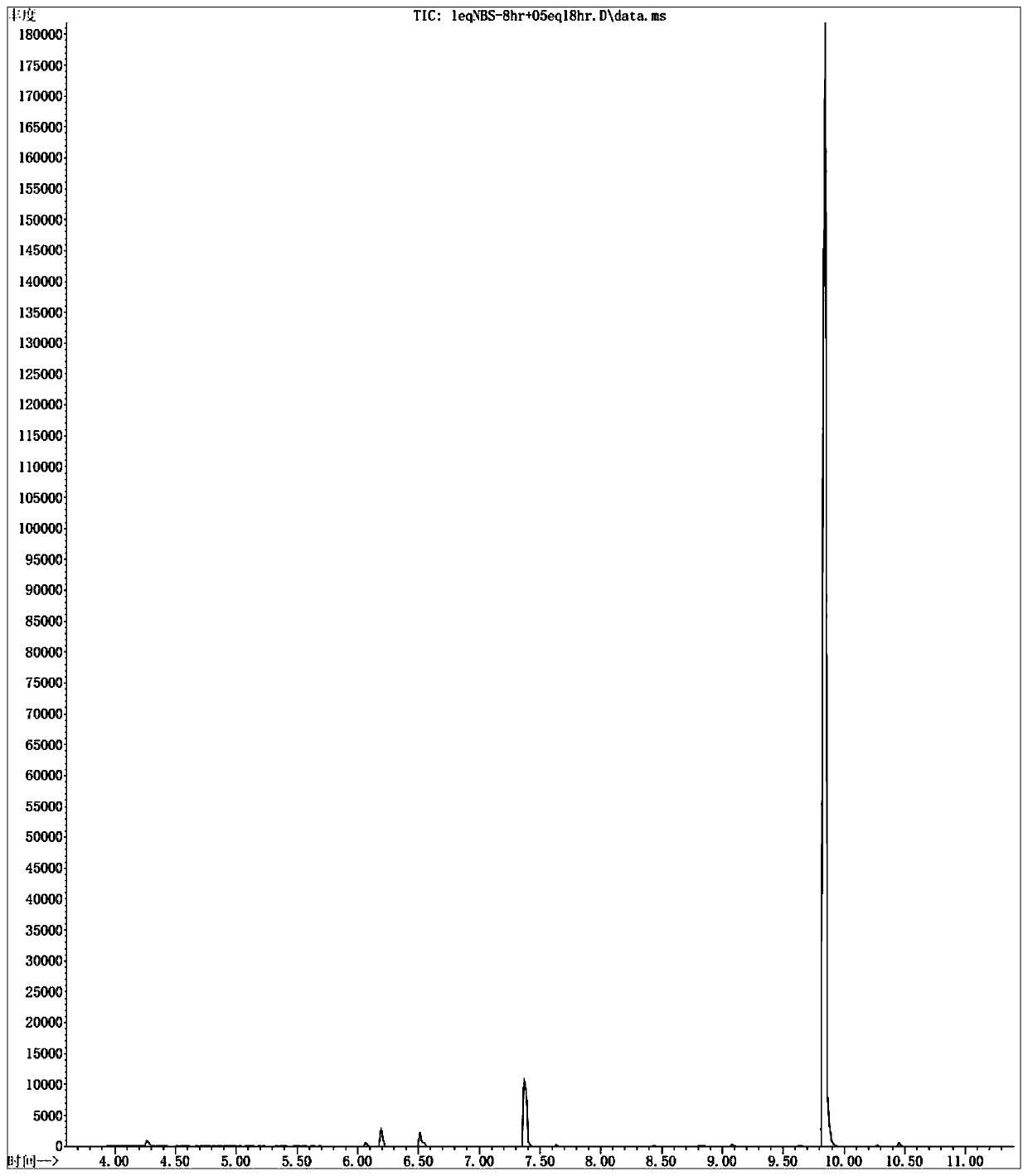
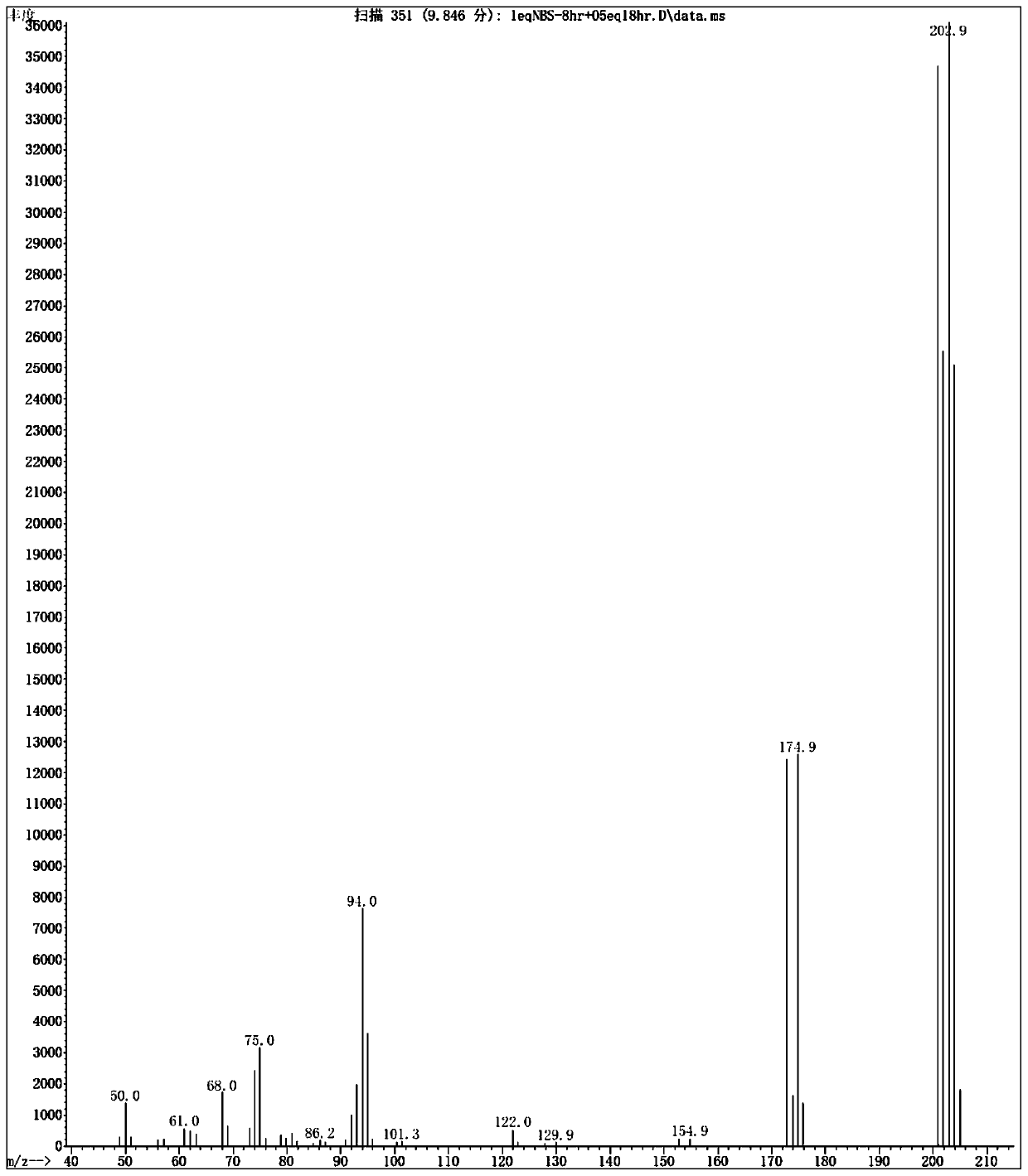
[0028] Add 100 mL of TFA / HO to a 250 mL two-neck flask 2 SO 4 Mixed solvent (volume ratio 5 / 1), down to 0 ° C, then add 12.4 grams (500 mmol) of 4-fluorobenzaldehyde, then rise to 50 ° C under stirring, add 14.3 grams (50 mmol) from the feeding port Dibromohydantoin solid, seal the feeding port after adding, keep the reaction system at 50°C and stir for 8 hours; open the feeding product, add 14.3 grams (50 mmol) of dibromohydantoin solid, keep stirring at 50°C for 48 hours . The reaction of the raw materials was complete, lowered to room temperature, the reaction solution was poured into 1L of ice water, the aqueous phase was extracted with n-hexane (100mL×3), the organic phase was combined, the organic phase was washed once with 20mL of saturated sodium bicarbonate solution, and 20mL of saturated chlorinated The sodium solution was washed once, and the n-hexane was removed by concentration under reduced pressure to obtain the crude product of 2-bromo-4-fluorobenzaldehyde. T...
Embodiment 2
[0035] Add 500 mL of TFA / HO to a 1 L three-necked flask 2 SO 4 Mix solvent (volume ratio 10 / 1), be down to 0 ℃, then add 62 grams (500 millimoles) 4-fluorobenzaldehyde, rise to 50 ℃ under stirring then, add 250 millimoles N-bromo For substituted succinimide solid, seal the feeding port after adding, keep the reaction system at 50°C and stir for 8 hours; open the feeding port, add 50 mmol N-bromosuccinimide solid, keep at 50°C Stir for 48 hours. After the reaction of the raw materials is complete, cool down to room temperature, slowly pour the reaction solution into 5 liters of ice water, extract the aqueous phase (500mL×3) with toluene, combine the organic phases, wash the organic phases with 100mL saturated sodium bicarbonate solution once, and wash with 100mL saturated sodium bicarbonate solution. The sodium chloride solution was washed once, the organic phase was concentrated under reduced pressure to remove toluene to obtain the crude product of 2-bromo-4-fluorobenzaldeh...
Embodiment 3
[0037] Into a 100 L reactor equipped with an electric stirrer unit was charged 50 L of TFA / H 2 SO 4Mixed solvent (volume ratio 5 / 1), down to 0 ° C, then add 6.24 kg (25 moles) of 4-fluorobenzaldehyde, then rise to 50 ° C under stirring, add 7.15 kg (25 moles) from the feeding port Dibromohydantoin solid, seal the feeding port after adding, keep the reaction system at 50°C and stir for 8 hours; open the feeding port, add 7.15 kg (50 mmol) of dibromohydantoin solid, keep stirring at 50°C for 48 hours . After the reaction of the raw materials is complete, cool down to room temperature, slowly pour the reaction solution into 500 liters of ice water, extract the aqueous phase (50L×3) with n-hexane, combine the organic phases, and wash the organic phases once with 10L saturated sodium bicarbonate solution, 10L The saturated sodium chloride solution was washed once, and the organic phase was concentrated under reduced pressure to remove n-hexane to obtain the crude product of 2-bro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com