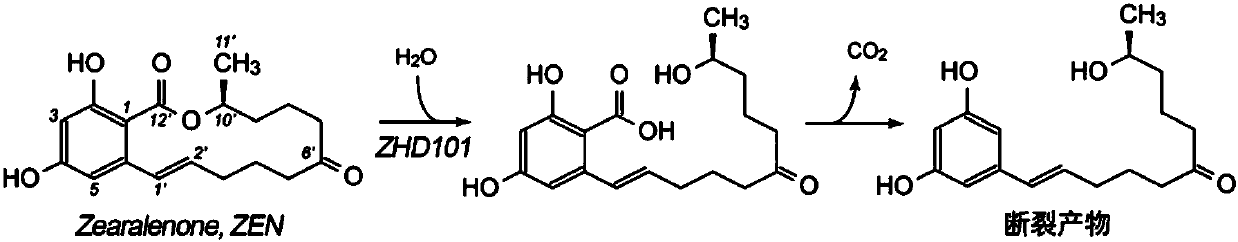
Zearalenone hydrolase ZHD101 mutant and method for hydrolyzing zearalenone by using same
A mutant and hydrolase technology, applied in the field of hydrolysis of zearalenone, can solve the problems of unsatisfactory catalytic efficiency, irreversible deactivation of the enzyme, etc., and achieve the effects of improved catalytic activity, high expression activity, and improved enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1, preparation and purification of ZHD101 mutant
[0065] 1. Construction of ZHD101 mutant coding gene and expression vector
[0066] The coding sequence (SEQ ID NO.2) of wild-type zearalenic ketohydrolase was replaced with the DNA sequence (SEQ ID NO.17) composed of Escherichia coli preferred codons, and corresponding mutations were introduced to obtain SEQ ID NO. 4. The nucleotide sequences shown in SEQ ID NO.6, SEQ ID NO.8, SEQ ID NO.10, SEQ ID NO.12, SEQ ID NO.14 and SEQ ID NO.16, at the 5' of the above sequence The Nde I restriction site CATATG was added to the end, and the DNA sequence CACCATCACCACCATCAC (SEQ ID NO.18) encoding the hexahistidine tag, the stop codon TAA and the Xho I restriction site CTCGAG were sequentially added to the 3' end, chemically synthesized The resulting DNA sequence (Invitrogen, USA). A histidine tag 6×His was added to the C-terminal end of the encoded protein sequence to facilitate subsequent purification steps. The resul...
Embodiment 2
[0080] Embodiment 2, the detection of the catalytic activity of ZHD101 mutant
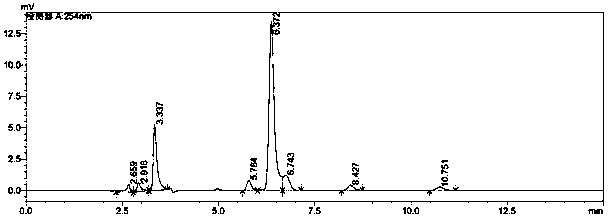
[0081] The target protein obtained in Step 3 of Example 1 was analyzed by HPLC (LC-20AT, Shimadzu Corporation), so as to determine the catalytic activity and kinetic parameters of the enzyme mutant. HPLC profile such as image 3 Shown (exemplarily shows the experimental results of ZHD101M3).
[0082] Specifically, the selected chromatographic column is a Hypersil C18 reverse-phase column (Elite, 5 μm, 4.6 mM×250 mM). The proportion of the reaction system is: 4850 μL sodium acetate (pH 5.5), 50 μL substrate (dissolved in acetonitrile, the concentration changes in a gradient, the highest concentration is 2 mg / mL), 100 μL enzyme solution (stored in 100 mM Tris buffer, pH 8.0 , concentration 100 μg / mL), and reacted for 10 min in a constant temperature water bath at 37°C. After the reaction, boil in boiling water for 1 min to terminate the reaction.
[0083] The mobile phase ratio (v / v) of HPLC is: ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com