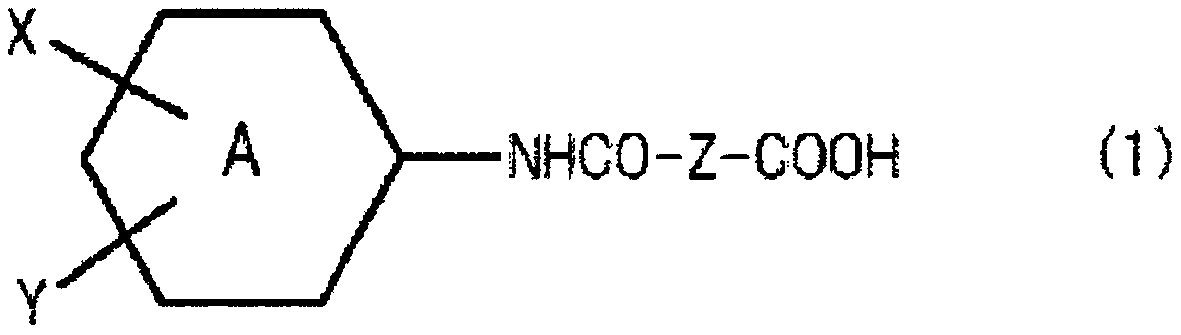Resist underlayer film-forming composition comprising amide group-containing polyester
A technology of resist lower layer and compound, applied in the direction of organic chemistry, photolithographic process of pattern surface, photosensitive material used in photomechanical equipment, etc., can solve the problem of resist pattern collapse, processing into desired shape or Size and other issues, to achieve the effect of dry etching speed, good cross-sectional shape, and sufficient anti-reflection function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0177] Hereinafter, the present invention will be described in further detail with reference to examples, but the present invention is not limited to the following aspects.
[0178] The identification of the compounds obtained in the following synthesis examples of this specification was carried out by NMR analysis. The apparatus used, measurement conditions, etc. are as follows.
[0179] Device: JNM-ECA500 manufactured by JEOL Ltd.
[0180] Nuclear species: proton
[0181] Temperature: 23°C
[0182] Frequency: 500MHz
[0183] Deuterated solvent: DMSO
[0184] The weight average molecular weight of the compound obtained in the following synthesis example of this specification is the measurement result by gel permeation chromatography (it abbreviates to GPC hereafter). The measurement apparatus, measurement conditions, and the like are as follows.
[0185] Device: HLC-8320GPC manufactured by Tosoh Corporation
[0186] GPC column: Asahipak [registered trademark] GF-310HQ,...
Synthetic example 1
[0192] Add 10.82 g of 1,4-phenylenediamine, 23.96 g of glutaric anhydride, and 139.02 g of tetrahydrofuran to a flask equipped with a stirrer, a thermometer, and a Dimrot cooling tube, and put the reaction solution into 300 ml of acetone to make the target substance Precipitate. The precipitate was filtered with a Kiriyama funnel, washed with acetone, and then dried under reduced pressure at 40° C. for 12 hours to obtain 31.27 g of a white powder (93% yield).
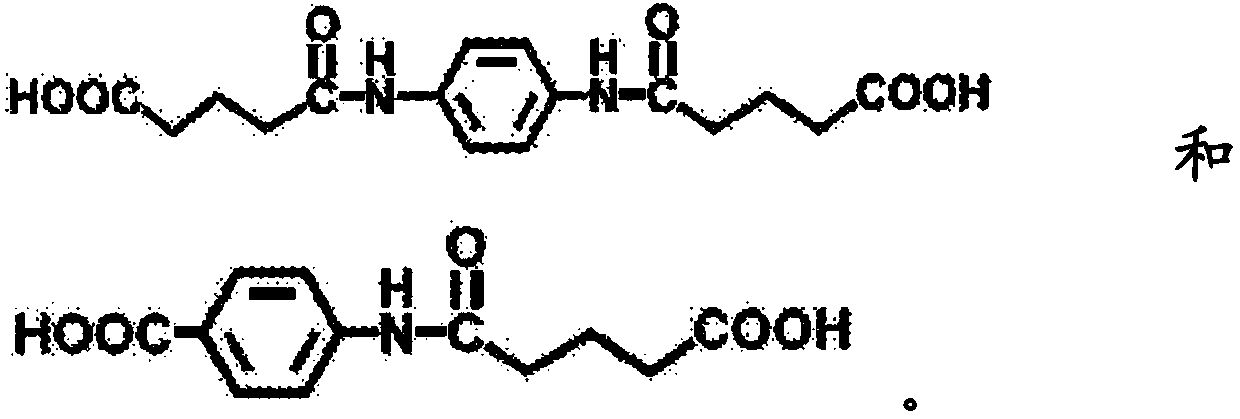
[0193] It was confirmed by NMR analysis that a compound (purity>95%) estimated to have the structure shown below was obtained. σ=1.79(4H, quin), 2.26(4H, t), 2.31(4H, t), 7.48(4H, D), 9, 80(2H, s), 12.06(2H, br)
[0194]
[0195] In the flask equipped with a stirrer, a thermometer, and a Dimrot cooling tube, 47.72 g of cyclohexanone (hereinafter referred to as CYH in this specification), 47.72 g of N,N-2-trimethylpropionamide (hereinafter , abbreviated as DMIB in this specification.) 11.93g, 6.60g of the obtained c...
Synthetic example 2
[0198] 10.81 g of 1,3-phenylenediamine, 23.97 g of glutaric anhydride, and 139.32 g of tetrahydrofuran were added to a flask equipped with a stirrer, a thermometer, and a Dimrot cooling tube, and heated to reflux for 2 hours under a nitrogen atmosphere. After completion of the reaction, the inside of the system was cooled down to room temperature, and then the reaction solution was poured into 300 ml of acetone to precipitate the target substance. The precipitate was filtered with a Kiriyama funnel, washed with acetone, and then dried under reduced pressure at 40° C. for 12 hours to obtain 22.69 g of a white powder (yield 71%).
[0199] It was confirmed by NMR analysis that a compound (purity>95%) estimated to have the structure shown below was obtained. σ=1.80(4H, quin), 2.27(4H, t), 2.33(4H, t), 7.17(1H, t), 7.25(2H, d), 7.92(1H, s), 9, 88(2H, s), 12.07 (2H, br)
[0200]
[0201] In a flask equipped with a stirrer, a thermometer, and a Dimrot cooling tube, 47.68 g of CY...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com